Perceptual learning improves contrast sensitivity of V1 neurons in cats
- PMID: 20451388
- PMCID: PMC2877770
- DOI: 10.1016/j.cub.2010.03.066
Perceptual learning improves contrast sensitivity of V1 neurons in cats
Abstract
Background: Perceptual learning has been documented in adult humans over a wide range of tasks. Although the often-observed specificity of learning is generally interpreted as evidence for training-induced plasticity in early cortical areas, physiological evidence for training-induced changes in early visual cortical areas is modest, despite reports of learning-induced changes of cortical activities in fMRI studies. To reveal the physiological bases of perceptual learning, we combined psychophysical measurements with extracellular single-unit recording under anesthetized preparations and examined the effects of training in grating orientation identification on both perceptual and neuronal contrast sensitivity functions of cats.
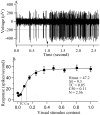
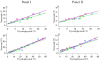
Results: We have found that training significantly improved perceptual contrast sensitivity of the cats to gratings with spatial frequencies near the "trained" spatial frequency, with stronger effects in the trained eye. Consistent with behavioral assessments, the mean contrast sensitivity of neurons recorded from V1 of the trained cats was significantly higher than that of neurons recorded from the untrained cats. Furthermore, in the trained cats, the contrast sensitivity of V1 neurons responding preferentially to stimuli presented via the trained eyes was significantly greater than that of neurons responding preferentially to stimuli presented via the "untrained" eyes. The effect was confined to the trained spatial frequencies. In both trained and untrained cats, the neuronal contrast sensitivity functions derived from the contrast sensitivity of the individual neurons were highly correlated with behaviorally determined perceptual contrast sensitivity functions.
Conclusions: We suggest that training-induced neuronal contrast gain in area V1 underlies behaviorally determined perceptual contrast sensitivity improvements.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures

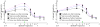
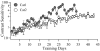

References
-
- Fiorentini A, Berardi N. Perceptual learning specific for orientation and spatial frequency. Nature. 1980;287:43–44. - PubMed
-
- Liu Z, Vaina LM. Simultaneous learning of motion discrimination in two directions. Cognitive Brain Research. 1998;6:347–349. - PubMed
-
- Sowden PT, Rose D, Davies IR. Perceptual learning of luminance contrast detection: specific for spatial frequency and retinal location but not orientation. Vision Res. 2002;42:1249–1258. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

