Minimal residual disease-directed therapy for childhood acute myeloid leukaemia: results of the AML02 multicentre trial
- PMID: 20451454
- PMCID: PMC3171799
- DOI: 10.1016/S1470-2045(10)70090-5
Minimal residual disease-directed therapy for childhood acute myeloid leukaemia: results of the AML02 multicentre trial
Abstract
Background: We sought to improve outcome in patients with childhood acute myeloid leukaemia (AML) by applying risk-directed therapy that was based on genetic abnormalities of the leukaemic cells and measurements of minimal residual disease (MRD) done by flow cytometry during treatment.
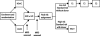
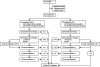
Methods: From Oct 13, 2002, to June 19, 2008, 232 patients with de-novo AML (n=206), therapy-related or myelodysplasia-related AML (n=12), or mixed-lineage leukaemia (n=14) were enrolled at eight centres. 230 patients were assigned by block, non-blinded randomisation, stratified by cytogenetic or morphological subtype, to high-dose (18 g/m(2), n=113) or low-dose (2 g/m(2), n=117) cytarabine given with daunorubicin and etoposide (ADE; induction 1). The primary aim of the study was to compare the incidence of MRD positivity of the high-dose group and the low-dose group at day 22 of induction 1. Induction 2 consisted of ADE with or without gemtuzumab ozogamicin (GO anti-CD33 monoclonal antibody); consolidation therapy included three additional courses of chemotherapy or haematopoietic stem-cell transplantation (HSCT). Levels of MRD were used to allocate GO and to determine the timing of induction 2. Both MRD and genetic abnormalities at diagnosis were used to determine the final risk classification. Low-risk patients (n=68) received five courses of chemotherapy, whereas high-risk patients (n=79), and standard-risk patients (n=69) with matched sibling donors, were eligible for HSCT (done for 48 high-risk and eight standard-risk patients). All 230 randomised patients were analysed for the primary endpoint. Other analyses were limited to the 216 patients with AML, excluding those with mixed-lineage leukaemia. This trial is closed to accrual and is registered with ClinicalTrials.gov, number NCT00136084.
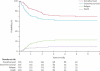
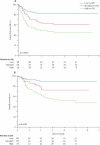
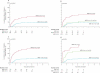
Findings: Complete remission was achieved in 80% (173 of 216 patients) after induction 1 and 94% (203 of 216) after induction 2. Induction failures included two deaths from toxic effects and ten cases of resistant leukaemia. The introduction of high-dose versus low-dose cytarabine did not significantly lower the rate of MRD-positivity after induction 1 (34%vs 42%, p=0.17). The 6-month cumulative incidence of grade 3 or higher infection was 79.3% (SE 4.0) for patients in the high-dose group and 75.5% (4.2) for the low-dose group. 3-year event-free survival and overall survival were 63.0% (SE 4.1) and 71.1% (3.8), respectively. 80% (155 of 193) of patients achieved MRD of less than 0.1% after induction 2, and the cumulative incidence of relapse for this group was 17% (SE 3). MRD of 1% or higher after induction 1 was the only significant independent adverse prognostic factor for both event-free (hazard ratio 2.41, 95% CI 1.36-4.26; p=0.003) and overall survival (2.11, 1.09-4.11; p=0.028).
Interpretation: Our findings suggest that the use of targeted chemotherapy and HSCT, in the context of a comprehensive risk-stratification strategy based on genetic features and MRD findings, can improve outcome in patients with childhood AML.
Funding: National Institutes of Health and American Lebanese Syrian Associated Charities (ALSAC).
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures
Comment in
-
Towards individualised treatment in childhood leukaemia.Lancet Oncol. 2010 Jun;11(6):502-3. doi: 10.1016/S1470-2045(10)70108-X. Lancet Oncol. 2010. PMID: 20522371 No abstract available.
References
-
- Creutzig U, Zimmermann M, Lehrnbecher T, et al. Less toxicity by optimizing chemotherapy, but not by addition of granulocyte colony-stimulating factor in children and adolescents with acute myeloid leukemia: results of AML-BFM 98. J Clin Oncol. 2006;24:4499–4506. - PubMed
-
- Tsukimoto I, Tawa A, Horibe K, et al. Risk-stratified therapy and the intensive use of cytarabine improves the outcome in childhood acute myeloid leukemia: the AML99 trial from the Japanese Childhood AML Cooperative Study Group. J Clin Oncol. 2009;27:4007–4013. - PubMed
-
- Grimwade D, Hills RK. Independent prognostic factors for AML outcome. Hematology Am Soc Hematol Educ Program. 2009:385–395. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

