Conserved helicase domain of human RecQ4 is required for strand annealing-independent DNA unwinding
- PMID: 20451470
- PMCID: PMC2893255
- DOI: 10.1016/j.dnarep.2010.04.003
Conserved helicase domain of human RecQ4 is required for strand annealing-independent DNA unwinding
Abstract
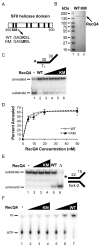
Humans have five members of the well conserved RecQ helicase family: RecQ1, Bloom syndrome protein (BLM), Werner syndrome protein (WRN), RecQ4, and RecQ5, which are all known for their roles in maintaining genome stability. BLM, WRN, and RecQ4 are associated with premature aging and cancer predisposition. Of the three, RecQ4's biological and cellular roles have been least thoroughly characterized. Here we tested the helicase activity of purified human RecQ4 on various substrates. Consistent with recent results, we detected ATP-dependent RecQ4 unwinding of forked duplexes. However, our results provide the first evidence that human RecQ4's unwinding is independent of strand annealing, and that it does not require the presence of excess ssDNA. Moreover, we demonstrate that a point mutation of the conserved lysine in the Walker A motif abolished helicase activity, implying that not the N-terminal portion, but the helicase domain is solely responsible for the enzyme's unwinding activity. In addition, we demonstrate a novel stimulation of RecQ4's helicase activity by replication protein A, similar to that of RecQ1, BLM, WRN, and RecQ5. Together, these data indicate that specific biochemical activities and protein partners of RecQ4 are conserved with those of the other RecQ helicases.
Published by Elsevier B.V.
Figures



Similar articles
-
Competition between the DNA unwinding and strand pairing activities of the Werner and Bloom syndrome proteins.BMC Mol Biol. 2006 Jan 13;7:1. doi: 10.1186/1471-2199-7-1. BMC Mol Biol. 2006. PMID: 16412221 Free PMC article.
-
The Human RecQ4 Helicase Contains a Functional RecQ C-terminal Region (RQC) That Is Essential for Activity.J Biol Chem. 2017 Mar 10;292(10):4176-4184. doi: 10.1074/jbc.M116.767954. Epub 2016 Dec 20. J Biol Chem. 2017. PMID: 27998982 Free PMC article.
-
Catalytic strand separation by RECQ1 is required for RPA-mediated response to replication stress.Curr Biol. 2015 Nov 2;25(21):2830-2838. doi: 10.1016/j.cub.2015.09.026. Epub 2015 Oct 8. Curr Biol. 2015. PMID: 26455304 Free PMC article.
-
Distinct roles of RECQ1 in the maintenance of genomic stability.DNA Repair (Amst). 2010 Mar 2;9(3):315-24. doi: 10.1016/j.dnarep.2009.12.010. Epub 2010 Jan 12. DNA Repair (Amst). 2010. PMID: 20061189 Free PMC article. Review.
-
Roles of Werner syndrome protein in protection of genome integrity.DNA Repair (Amst). 2010 Mar 2;9(3):331-44. doi: 10.1016/j.dnarep.2009.12.011. Epub 2010 Jan 13. DNA Repair (Amst). 2010. PMID: 20075015 Free PMC article. Review.
Cited by
-
RECQL5 cooperates with Topoisomerase II alpha in DNA decatenation and cell cycle progression.Nucleic Acids Res. 2012 Feb;40(4):1621-35. doi: 10.1093/nar/gkr844. Epub 2011 Oct 19. Nucleic Acids Res. 2012. PMID: 22013166 Free PMC article.
-
Senescence induced by RECQL4 dysfunction contributes to Rothmund-Thomson syndrome features in mice.Cell Death Dis. 2014 May 15;5(5):e1226. doi: 10.1038/cddis.2014.168. Cell Death Dis. 2014. PMID: 24832598 Free PMC article.
-
RECQ1 is required for cellular resistance to replication stress and catalyzes strand exchange on stalled replication fork structures.Cell Cycle. 2012 Nov 15;11(22):4252-65. doi: 10.4161/cc.22581. Epub 2012 Oct 24. Cell Cycle. 2012. PMID: 23095637 Free PMC article.
-
The Rothmund-Thomson syndrome helicase RECQL4 is essential for hematopoiesis.J Clin Invest. 2014 Aug;124(8):3551-65. doi: 10.1172/JCI75334. Epub 2014 Jun 24. J Clin Invest. 2014. PMID: 24960165 Free PMC article.
-
Interaction of RECQ4 and MCM10 is important for efficient DNA replication origin firing in human cells.Oncotarget. 2015 Dec 1;6(38):40464-79. doi: 10.18632/oncotarget.6342. Oncotarget. 2015. PMID: 26588054 Free PMC article.
References
-
- Bachrati CZ, Hickson ID. RecQ helicases: guardian angels of the DNA replication fork. Chromosoma. 2008;117:219–233. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

