Influenza M2 proton channels
- PMID: 20451491
- PMCID: PMC3108042
- DOI: 10.1016/j.bbamem.2010.04.015
Influenza M2 proton channels
Abstract
M2 of the influenza virus is an intriguing transmembrane protein that forms a minuscule proton channel in the viral envelope. Its recognized function is to equilibrate pH across the viral membrane during cell entry and across the trans-Golgi membrane of infected cells during viral maturation. It is vital for viral replication and it is a target for the anti-influenza drugs, amantadine and rimantadine. Recently, high resolution structures of M2 channels of both flu A and B have been obtained, providing the desperately needed structural details for understanding the mechanism of proton conductance. In particular, the establishment of the functional solution NMR system of the proton channels enabled simultaneous high resolution structure characterization and measurement of channel dynamics coupled to channel activity. This review summarizes our current understanding of how protons are conducted through the M2 channel from a structural point of view, as well as the modes by which important channel gating elements function during proton conduction.
2010 Elsevier B.V. All rights reserved.
Figures
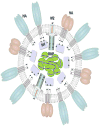

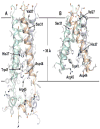
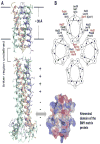
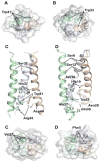
References
-
- Palese P, Shaw ML. Orthomyxoviridae: the viruses and their replication. In: Knipe DM, Howley PM, editors. Fields virology. Williams & Wilkins; 2007.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

