Murine intracochlear drug delivery: reducing concentration gradients within the cochlea
- PMID: 20451593
- PMCID: PMC2933796
- DOI: 10.1016/j.heares.2010.04.014
Murine intracochlear drug delivery: reducing concentration gradients within the cochlea
Abstract
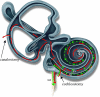
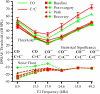
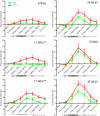
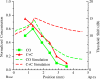
Direct delivery of compounds to the mammalian inner ear is most commonly achieved by absorption or direct injection through the round window membrane (RWM), or infusion through a basal turn cochleostomy. These methods provide direct access to cochlear structures, but with a strong basal-to-apical concentration gradient consistent with a diffusion-driven distribution. This gradient limits the efficacy of therapeutic approaches for apical structures, and puts constraints on practical therapeutic dose ranges. A surgical approach involving both a basal turn cochleostomy and a posterior semicircular canal canalostomy provides opportunities for facilitated perfusion of cochlear structures to reduce concentration gradients. Infusion of fixed volumes of artificial perilymph (AP) and sodium salicylate were used to evaluate two surgical approaches in the mouse: cochleostomy-only (CO), or cochleostomy-plus-canalostomy (C+C). Cochlear function was evaluated via closed-system distortion product otoacoustic emissions (DPOAE) threshold level measurements from 8 to 49 kHz. AP infusion confirmed no surgical impact to auditory function, while shifts in DPOAE thresholds were measured during infusion of salicylate and AP (washout). Frequency dependent shifts were compared for the CO and C+C approaches. Computer simulations modeling diffusion, volume flow, interscala transport, and clearance mechanisms provided estimates of drug concentration as a function of cochlear position. Simulated concentration profiles were compared to frequency-dependent shifts in measured auditory responses using a cochlear tonotopic map. The impact of flow rate on frequency dependent DPOAE threshold shifts was also evaluated for both surgical approaches. Both the C+C approach and a flow rate increase were found to provide enhanced response for lower frequencies, with evidence suggesting the C+C approach reduces concentration gradients within the cochlea.
Copyright (c) 2010 Elsevier B.V. All rights reserved.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

