Linear and conformation specific antibodies in aged beagles after prolonged vaccination with aggregated Abeta
- PMID: 20451612
- PMCID: PMC2910127
- DOI: 10.1016/j.nbd.2010.04.014
Linear and conformation specific antibodies in aged beagles after prolonged vaccination with aggregated Abeta
Abstract
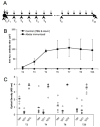
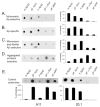
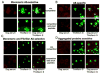
Previously we showed that anti-Abeta peptide immunotherapy significantly attenuated Alzheimer's-like amyloid deposition in the central nervous system of aged canines. In this report we have characterized the changes that occurred in the humoral immune response over 2.4years in canines immunized repeatedly with aggregated Abeta(1-42) (AN1792) formulated in alum adjuvant. We observed a rapid and robust induction of anti-Abeta antibody titers, which were associated with an anti-inflammatory T helper type 2 (Th2) response. The initial antibody response was against dominant linear epitope at the N-terminus region of the Abeta(1-42) peptide, which is identical to the one in humans and vervet monkeys. After multiple immunizations the antibody response drifted toward the elevation of antibodies that recognized conformational epitopes of assembled forms of Abeta and other types of amyloid. Our findings indicate that prolonged immunization results in distinctive temporal changes in antibody profiles, which may be important for other experimental and clinical settings.
Figures




References
-
- Anguiano M, et al. Protofibrillar islet amyloid polypeptide permeabilizes synthetic vesicles by a pore-like mechanism that may be relevant to type II diabetes. Biochemistry. 2002;41:11338–43. - PubMed
-
- Bayer AJ, et al. Evaluation of the safety and immunogenicity of synthetic Abeta42 (AN1792) in patients with AD. Neurology. 2005;64:94–101. - PubMed
-
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AG031764/AG/NIA NIH HHS/United States
- AG20241/AG/NIA NIH HHS/United States
- AG20242/AG/NIA NIH HHS/United States
- P01 AG000538/AG/NIA NIH HHS/United States
- P50 AG16573/AG/NIA NIH HHS/United States
- P50 AG016573/AG/NIA NIH HHS/United States
- RF1 AG020241/AG/NIA NIH HHS/United States
- R01 NS050895/NS/NINDS NIH HHS/United States
- AG00538/AG/NIA NIH HHS/United States
- R01 AG020241/AG/NIA NIH HHS/United States
- NS50895/NS/NINDS NIH HHS/United States
- R01 AG031764/AG/NIA NIH HHS/United States
- R01 AG020242/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

