Long-lasting humoral and cellular immune responses and mucosal dissemination after intramuscular DNA immunization
- PMID: 20451642
- PMCID: PMC2932451
- DOI: 10.1016/j.vaccine.2010.04.064
Long-lasting humoral and cellular immune responses and mucosal dissemination after intramuscular DNA immunization
Abstract
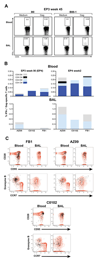
Naïve Indian rhesus macaques were immunized with a mixture of optimized plasmid DNAs expressing several SIV antigens using in vivo electroporation via the intramuscular route. The animals were monitored for the development of SIV-specific systemic (blood) and mucosal (bronchoalveolar lavage) cellular and humoral immune responses. The immune responses were of great magnitude, broad (Gag, Pol, Nef, Tat and Vif), long-lasting (up to 90 weeks post third vaccination) and were boosted with each subsequent immunization, even after an extended 90-week rest period. The SIV-specific cellular immune responses were consistently more abundant in bronchoalveolar lavage (BAL) than in blood, and were characterized as predominantly effector memory CD4(+) and CD8(+) T cells in BAL and as both central and effector memory T cells in blood. SIV-specific T cells containing Granzyme B were readily detected in both blood and BAL, suggesting the presence of effector cells with cytolytic potential. DNA vaccination also elicited long-lasting systemic and mucosal humoral immune responses, including the induction of Gag-specific IgA. The combination of optimized DNA vectors and improved intramuscular delivery by in vivo electroporation has the potential to elicit both cellular and humoral responses and dissemination to the periphery, and thus to improve DNA immunization efficacy.
Published by Elsevier Ltd.
Figures





References
-
- Paiardini M, Frank I, Pandrea I, Apetrei C, Silvestri G. Mucosal immune dysfunction in AIDS pathogenesis. AIDS Rev. 2008 Jan–Mar;10(1):36–46. - PubMed
-
- Belyakov IM, Berzofsky JA. Immunobiology of mucosal HIV infection and the basis for development of a new generation of mucosal AIDS vaccines. Immunity. 2004 Mar;20(3):247–253. - PubMed
-
- Yuki Y, Nochi T, Kiyono H. Progress towards an AIDS mucosal vaccine: an overview. Tuberculosis (Edinb) 2007 Aug;87 Suppl 1:S35–S44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

