Lyso-phosphatidylcholine induces osteogenic gene expression and phenotype in vascular smooth muscle cells
- PMID: 20451909
- PMCID: PMC2902706
- DOI: 10.1016/j.atherosclerosis.2010.04.005
Lyso-phosphatidylcholine induces osteogenic gene expression and phenotype in vascular smooth muscle cells
Abstract
Objective: Calcifying vascular cells in human atherosclerotic plaques actively contribute to ectopic vascular mineralization. Lyso-phosphatidylcholine (LPC), a product of oxidized phosphatidylcholine hydrolysis, is found at concentrations of 1-12 microg/g tissue throughout the atheroma. The objective of this study was to determine if LPC induces an osteogenic phenotype in vascular smooth muscle cells.
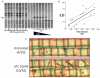
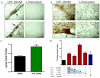
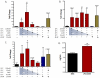
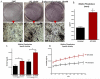
Methods and results: Proliferating human aortic smooth muscle cells were treated with a wide-range of LPC concentrations (0.1 nM to 100 microM) over 14 days. Von Kossa, Alizarin Red S, and alkaline phosphatase staining were used to identify mineralizations. RT-PCR, ELISA, alkaline phosphatase activity, and 45Ca incorporation assays were used to evaluate the osteo-inductive effect of LPC on smooth muscle phenotype. Histology and morphometry revealed that cells treated with as little as 10 nM LPC produced calcium phosphate deposits in culture. LPC-treated vascular smooth muscle cells showed a significant increase in 45Ca incorporation and alkaline phosphatase activity. Furthermore, LPC treatment induced a significant loss of Schnurri 3 protein, a key repressor of Runt-related transcription factor 2 stability. Genomic studies revealed that osteogenic gene expression was significantly up-regulated in LPC-treated cells, which is attributed to increased Runt-related transcription factor 2 expression and transcriptional activity.
Conclusion: LPC induces osteogenic morphology, physiology, gene expression, and phenotype in vascular smooth muscle cells. The present study suggests that localized concentrations of LPC in human atherosclerotic plaques may be a contributing factor to the generation of calcifying vascular cells.
Copyright (c) 2010 Elsevier Ireland Ltd. All rights reserved.
Figures
Comment in
-
Lysophospholipids: effectors mediating the contribution of dyslipidemia to calcification associated with atherosclerosis.Atherosclerosis. 2010 Jul;211(1):36-7. doi: 10.1016/j.atherosclerosis.2010.02.005. Epub 2010 Feb 10. Atherosclerosis. 2010. PMID: 20197191 No abstract available.
Similar articles
-
Effect of lyso-phosphatidylcholine and Schnurri-3 on osteogenic transdifferentiation of vascular smooth muscle cells to calcifying vascular cells in 3D culture.Biochim Biophys Acta. 2013 Jun;1830(6):3828-34. doi: 10.1016/j.bbagen.2013.02.015. Epub 2013 Mar 14. Biochim Biophys Acta. 2013. PMID: 23500015 Free PMC article. Clinical Trial.
-
Smooth muscle cell phenotypic transition associated with calcification: upregulation of Cbfa1 and downregulation of smooth muscle lineage markers.Circ Res. 2001 Dec 7;89(12):1147-54. doi: 10.1161/hh2401.101070. Circ Res. 2001. PMID: 11739279
-
Diosgenin stimulates osteogenic activity by increasing bone matrix protein synthesis and bone-specific transcription factor Runx2 in osteoblastic MC3T3-E1 cells.J Nutr Biochem. 2011 Nov;22(11):1055-63. doi: 10.1016/j.jnutbio.2010.09.003. Epub 2011 Feb 2. J Nutr Biochem. 2011. PMID: 21292464
-
Inhibition of osteo/chondrogenic transformation of vascular smooth muscle cells by MgCl2 via calcium-sensing receptor.J Hypertens. 2017 Mar;35(3):523-532. doi: 10.1097/HJH.0000000000001202. J Hypertens. 2017. PMID: 27984337
-
Overexpression of c1q/tumor necrosis factor-related protein-3 promotes phosphate-induced vascular smooth muscle cell calcification both in vivo and in vitro.Arterioscler Thromb Vasc Biol. 2014 May;34(5):1002-10. doi: 10.1161/ATVBAHA.114.303301. Epub 2014 Feb 27. Arterioscler Thromb Vasc Biol. 2014. PMID: 24578384
Cited by
-
The Interplay of Lipid Signaling in Musculoskeletal Cross Talk: Implications for Health and Disease.Methods Mol Biol. 2024;2816:1-11. doi: 10.1007/978-1-0716-3902-3_1. Methods Mol Biol. 2024. PMID: 38977583
-
Lp(a) in the Pathogenesis of Aortic Stenosis and Approach to Therapy with Antisense Oligonucleotides or Short Interfering RNA.Int J Mol Sci. 2023 Oct 6;24(19):14939. doi: 10.3390/ijms241914939. Int J Mol Sci. 2023. PMID: 37834387 Free PMC article. Review.
-
Escaping the cut by restriction enzymes through single-strand self-annealing of host-edited 12-bp and longer synthetic palindromes.DNA Cell Biol. 2012 Feb;31(2):151-63. doi: 10.1089/dna.2011.1339. Epub 2011 Sep 6. DNA Cell Biol. 2012. PMID: 21895510 Free PMC article.
-
Distribution of alkaline phosphatase, osteopontin, RANK ligand and osteoprotegerin in calcified human carotid atheroma.Protein J. 2015 Oct;34(5):315-28. doi: 10.1007/s10930-015-9620-3. Protein J. 2015. PMID: 26307009
-
The roles of lipid oxidation products and receptor activator of nuclear factor-κB signaling in atherosclerotic calcification.Circ Res. 2011 Jun 10;108(12):1482-93. doi: 10.1161/CIRCRESAHA.110.234245. Circ Res. 2011. PMID: 21659652 Free PMC article. Review.
References
-
- Abedin M, Tintut Y, Demer LL. Vascular calcification: mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol. 2004;24:1161–1170. - PubMed
-
- Sangiorgi G, Rumberger JA, Severson A, et al. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: a histologic study of 723 coronary artery segments using nondecalcifying methodology. J Am Coll Cardiol. 1998;31:126–133. - PubMed
-
- Speer MY, Giachelli CM. Regulation of cardiovascular calcification. Cardiovasc Pathol. 2004;13:63–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

