Ovalbumin content of influenza vaccines
- PMID: 20451987
- PMCID: PMC2927356
- DOI: 10.1016/j.jaci.2010.03.009
Ovalbumin content of influenza vaccines
Figures
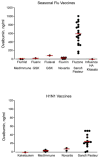
Comment on
-
Ovalbumin content in 2009 to 2010 seasonal and H1N1 monovalent influenza vaccines.J Allergy Clin Immunol. 2010 Mar;125(3):749-51, 751.e1. doi: 10.1016/j.jaci.2009.12.015. Epub 2010 Jan 8. J Allergy Clin Immunol. 2010. PMID: 20060576 No abstract available.
Similar articles
-
Prevention: Vaccine for all seasons.Nature. 2011 Nov 7;480(7376):S6-8. doi: 10.1038/480S6a. Nature. 2011. PMID: 22158298 No abstract available.
-
[Immunogenicity for different formulation of influenza A (H1N1) 2009 vaccines: a meta-analysis of seven randomized clinical trials].Zhonghua Liu Xing Bing Xue Za Zhi. 2010 May;31(5):590-2. Zhonghua Liu Xing Bing Xue Za Zhi. 2010. PMID: 22993766 Chinese. No abstract available.
-
Influenza immunization in egg allergy: an update for the 2011-2012 season.Clin Exp Allergy. 2011 Oct;41(10):1367-70. doi: 10.1111/j.1365-2222.2011.03842.x. Clin Exp Allergy. 2011. PMID: 22073922 Review.
-
Influenza vaccination: lessons learned from the pandemic (H1N1) 2009 influenza outbreak.Mucosal Immunol. 2010 Sep;3(5):422-4. doi: 10.1038/mi.2010.34. Epub 2010 Jun 16. Mucosal Immunol. 2010. PMID: 20555316 No abstract available.
-
Safety of seasonal influenza and influenza A (H1N1) 2009 monovalent vaccines in pregnancy.Expert Rev Vaccines. 2012 Aug;11(8):911-21. doi: 10.1586/erv.12.72. Expert Rev Vaccines. 2012. PMID: 23002972 Review.
Cited by
-
Effectiveness and safety of seasonal influenza vaccination in children with underlying respiratory diseases and allergy.Korean J Pediatr. 2014 Apr;57(4):164-70. doi: 10.3345/kjp.2014.57.4.164. Epub 2014 Apr 30. Korean J Pediatr. 2014. PMID: 24868213 Free PMC article. Review.
-
Current and Novel Approaches in Influenza Management.Vaccines (Basel). 2019 Jun 18;7(2):53. doi: 10.3390/vaccines7020053. Vaccines (Basel). 2019. PMID: 31216759 Free PMC article. Review.
-
[Health Technology Assessment: a value-based tool for the evaluation of healthcare technologies. Reassessment of the cell-culture-derived quadrivalent influenza vaccine: Flucelvax Tetra® 2.0].J Prev Med Hyg. 2023 Mar 2;63(4 Suppl 1):E1-E140. doi: 10.15167/2421-4248/jpmh2022.63.4s1. eCollection 2022 Dec. J Prev Med Hyg. 2023. PMID: 37034835 Free PMC article. Italian. No abstract available.
-
Statement on Seasonal Influenza Vaccine for 2011-2012: An Advisory Committee Statement (ACS) National Advisory Committee on Immunization (NACI)†.Can Commun Dis Rep. 2011 Oct 14;37(ACS-5):1-55. doi: 10.14745/ccdr.v37i00a05. eCollection 2011 Oct 14. Can Commun Dis Rep. 2011. PMID: 31682646 Free PMC article. No abstract available.
-
Vaccination in children with allergy to non active vaccine components.Clin Transl Med. 2015 Feb 14;4:3. doi: 10.1186/s40169-014-0043-0. eCollection 2015. Clin Transl Med. 2015. PMID: 25852819 Free PMC article.
References
-
- Rank MA, Li JT. Clinical pearls for preventing, diagnosing, and treating seasonal and 2009 H1N1 influenza infection in patients with asthma. J Allerg Clin Immunol. 2009;124:1123–6. - PubMed
-
- Chaloupka I, Schuler A, Marschall M. Meier-Ewert H Comparative analysis of six European influenza vaccines. Eur J Clin Microbiol Inf Dis. 1996;15:121–7. - PubMed
-
- Zeiger RS. Current issues with influenza vaccination in egg allergy. J Allerg Clin Immunol. 2002;110:834–40. - PubMed
-
- Waibel KH, Gomez R. Ovalbumin content in 2009 to 2010 seasonal and H1N1 monovalent influenza vaccines. J All Clin Immunol. 2010 in press. - PubMed
-
- James JM, Zeiger RS, Lester MR, Fasano MB, Gern JE, Mansfield LE, et al. Safe administration of influenza vaccine to patients with egg allergy. J Pediatr. 1998;133:624–8. - PubMed

