Peripheral nerve and brain differ in their capacity to resolve N,N-diethyldithiocarbamate-mediated elevations in copper and oxidative injury
- PMID: 20452388
- PMCID: PMC2900428
- DOI: 10.1016/j.tox.2010.04.018
Peripheral nerve and brain differ in their capacity to resolve N,N-diethyldithiocarbamate-mediated elevations in copper and oxidative injury
Abstract
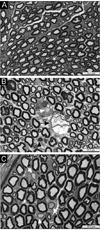
Previous studies have demonstrated that N,N-diethyldithiocarbamate (DEDC) elevates copper and promotes oxidative stress within the nervous system. However, whether these effects resolve following cessation of exposure or have the potential to persist and result in cumulative injury has not been determined. In this study, an established model for DEDC myelin injury in the rat was used to determine whether copper levels, oxidative stress, and neuromuscular deficits resolve following the cessation of DEDC exposure. Rats were exposed to DEDC for 8 weeks and then either euthanized or maintained for 2, 6 or 12 weeks after cessation of exposure. At each time point copper levels were measured by inductively coupled mass spectrometry to assess the ability of sciatic nerve, brain, spinal cord and liver to eliminate excess copper post-exposure. The protein expression levels of glutathione transferase alpha, heme oxygenase 1 and superoxide dismutase 1 in peripheral nerve and brain were also determined by western blot to assess levels of oxidative stress as a function of post-exposure duration. As an initial assessment of the bioavailability of the excess copper in brain the protein expression levels of copper chaperone for superoxide dismutase 1, and prion protein were determined by western blot as a function of exposure and post-exposure duration. Neuromuscular function in peripheral nerve was evaluated using grip strengths, nerve conduction velocities, and morphologic changes at the light microscope level. The data demonstrated that in peripheral nerve, copper levels and oxidative stress return to control levels within several weeks after cessation of exposure. Neuromuscular function also showed a trend towards pre-exposure values, although the resolution of myelin lesions was more delayed. In contrast, total copper and antioxidant enzyme levels remained significantly elevated in brain for longer post-exposure periods. The persistence of effects observed in brain suggests that the central nervous system is more susceptible to long-term cumulative adverse effects from dithiocarbamates. Additionally, significant changes in expression levels of chaperone for superoxide dismutase 1, and prion protein were observed consistent with at least a portion of the excess copper being bioactive.
2010 Elsevier Ireland Ltd. All rights reserved.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures




Similar articles
-
N,N-diethyldithiocarbamate promotes oxidative stress prior to myelin structural changes and increases myelin copper content.Toxicol Appl Pharmacol. 2009 Aug 15;239(1):71-9. doi: 10.1016/j.taap.2009.05.017. Epub 2009 May 23. Toxicol Appl Pharmacol. 2009. PMID: 19467251 Free PMC article.
-
Nitrogen substituent polarity influences dithiocarbamate-mediated lipid oxidation, nerve copper accumulation, and myelin injury.Chem Res Toxicol. 2009 Jan;22(1):218-26. doi: 10.1021/tx8003714. Chem Res Toxicol. 2009. PMID: 19093748 Free PMC article.
-
N,N-diethyldithiocarbamate produces copper accumulation, lipid peroxidation, and myelin injury in rat peripheral nerve.Toxicol Sci. 2004 Sep;81(1):160-71. doi: 10.1093/toxsci/kfh190. Epub 2004 Jun 8. Toxicol Sci. 2004. PMID: 15187237
-
Copper accumulation and lipid oxidation precede inflammation and myelin lesions in N,N-diethyldithiocarbamate peripheral myelinopathy.Toxicol Appl Pharmacol. 2008 May 15;229(1):77-85. doi: 10.1016/j.taap.2008.01.005. Epub 2008 Jan 26. Toxicol Appl Pharmacol. 2008. PMID: 18284930 Free PMC article.
-
Peripheral nerve protein expression and carbonyl content in N,N-diethlydithiocarbamate myelinopathy.Chem Res Toxicol. 2007 Mar;20(3):370-9. doi: 10.1021/tx6003453. Epub 2007 Feb 27. Chem Res Toxicol. 2007. PMID: 17323979 Free PMC article.
Cited by
-
Ziram and sodium N,N-dimethyldithiocarbamate inhibit ubiquitin activation through intracellular metal transport and increased oxidative stress in HEK293 cells.Chem Res Toxicol. 2015 Apr 20;28(4):682-90. doi: 10.1021/tx500450x. Epub 2015 Mar 23. Chem Res Toxicol. 2015. PMID: 25714994 Free PMC article.
-
Scutellarin Alleviates Cuprizone-Induced Demyelination by Improving Mitochondrial Dysfunction, Reducing Lipid Oxidation and Inhibiting the p38 MAPK Pathway.Antioxidants (Basel). 2025 Jun 12;14(6):723. doi: 10.3390/antiox14060723. Antioxidants (Basel). 2025. PMID: 40563355 Free PMC article.
-
Dopaminergic neurotoxicity of S-ethyl N,N-dipropylthiocarbamate (EPTC), molinate, and S-methyl-N,N-diethylthiocarbamate (MeDETC) in Caenorhabditis elegans.J Neurochem. 2013 Dec;127(6):837-51. doi: 10.1111/jnc.12349. Epub 2013 Jul 19. J Neurochem. 2013. PMID: 23786526 Free PMC article.
-
Dietary Copper Reduces the Hepatotoxicity of (-)-Epigallocatechin-3-Gallate in Mice.Molecules. 2017 Dec 23;23(1):38. doi: 10.3390/molecules23010038. Molecules. 2017. PMID: 29295524 Free PMC article.
References
-
- Aaseth J, Soli NE, Forre O. Increased brain uptake of copper and zinc in mice caused by diethyldithiocarbamate. Acta Pharmacol Toxicol. 1979;45:41–44. - PubMed
-
- Aschner M. The functional significance of brain metallothioneins. Faseb J. 1996;10:1129–1136. - PubMed
-
- Awasthi YC, Ansari GA, Awasthi S. Regulation of 4-hydroxynonenal mediated signaling by glutathione s-transferases. Methods Enzymol. 2005;401:379–407. - PubMed
-
- Betz WC, Goldstein GW, Katzman R. In: Blood-brain-cerebrospinal fluid barriers. Siegel GJ, Agranoff BW, Albers RW, Molinoff PB, editors. New York: Basic Neurochemistry, Raven Press, Ltd; 1994. pp. 681–699.
-
- Calviello G, Filippi GM, Toesca A, Palozza P, Maggiano N, Nicuolo FD, Serini S, Azzena GB, Galeotti T. Repeated exposure to pyrrolidine-dithiocarbamate induces peripheral nerve alterations in rats. Toxicol Lett. 2005;158:61–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

