Pharmacologic actions of progestins to inhibit cervical ripening and prevent delivery depend on their properties, the route of administration, and the vehicle
- PMID: 20452487
- PMCID: PMC3048062
- DOI: 10.1016/j.ajog.2010.03.025
Pharmacologic actions of progestins to inhibit cervical ripening and prevent delivery depend on their properties, the route of administration, and the vehicle
Abstract
Objective: The purpose of this study was to evaluate cervical changes and delivery at term during pregnancy in rats after various progestin treatments.
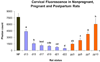
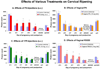
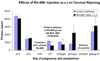
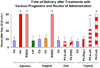
Study design: Pregnant rats were treated by various routes and vehicles with progesterone, 17-alpha-hydroxyprogesterone caproate (17P), R5020, and RU-486. Delivery time was determined and cervical ripening was assessed in vivo by collagen light-induced fluorescence.
Results: The cervix is rigid in the progesterone injection, 17P, and vaginal R5020 groups vs controls. Vaginal progesterone had no effect. RU-486 treatment softened the cervix during preterm delivery. Only subcutaneous injected progesterone, R5020 (subcutaneous and vaginal), and topical progesterone in sesame and fish oil inhibits delivery. Delivery is not changed by subcutaneous injection of 17P, vaginal progesterone, oral progesterone, and topical progesterone in Replens (Crinone; Columbia Labs, Livingston, NJ).
Conclusion: Inhibition of cervical ripening and delivery by progestins depends on many factors that include their properties, the route of administration, and the vehicle. This study suggests reasons that the present treatments for preterm labor are not efficacious.
Copyright (c) 2010 Mosby, Inc. All rights reserved.
Figures

References
-
- Steer P. The epidemiology of preterm labour. BJOG. 2005;112 Suppl 1:1–3. - PubMed
-
- Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S, Mathews TJ. Births: Final Data for 2006. Natl Vital Stat Rep. 2009;57:1–104. - PubMed
-
- Berkowitz GS, Papiernik E. Epidemiology of preterm birth. Epidemiol Rev. 1993;15(2):414–443. - PubMed
-
- Behrman R, Butler A. Preterm Birth: Causes, Consequences, and Prevention. Washington, D.C: The National Academies Press; 2007. pp. 398–429. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

