Prostate-specific membrane antigen-targeted photodynamic therapy induces rapid cytoskeletal disruption
- PMID: 20452720
- PMCID: PMC3201799
- DOI: 10.1016/j.canlet.2010.04.003
Prostate-specific membrane antigen-targeted photodynamic therapy induces rapid cytoskeletal disruption
Abstract
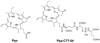
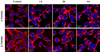
Prostate-specific membrane antigen (PSMA), an established enzyme-biomarker for prostate cancer, has attracted considerable attention as a target for imaging and therapeutic applications. We aimed to determine the effects of PSMA-targeted photodynamic therapy (PDT) on cytoskeletal networks in prostate cancer cells. PSMA-targeted PDT resulted in rapid disruption of microtubules (alpha-/beta-tubulin), microfilaments (actin), and intermediate filaments (cytokeratin 8/18) in the cytoplasm of LNCaP cells. The collapse of cytoplasmic microtubules and the later nuclear translocation of alpha-/beta-tubulin were the most dramatic alternation. It is likely that these early changes of cytoskeletal networks are partly involved in the initiation of cell death.
Copyright 2010 Elsevier Ireland Ltd. All rights reserved.
Conflict of interest statement
Dr. Berkman is the inventor of a patent on the PSMA inhibitor described in this report and presently serves as the CSO of Cancer Targeted Technology.
Figures



Similar articles
-
In vitro targeted photodynamic therapy with a pyropheophorbide--a conjugated inhibitor of prostate-specific membrane antigen.Prostate. 2009 May 1;69(6):585-94. doi: 10.1002/pros.20909. Prostate. 2009. PMID: 19142895 Free PMC article.
-
Targeted photodynamic therapy for prostate cancer: inducing apoptosis via activation of the caspase-8/-3 cascade pathway.Int J Oncol. 2010 Apr;36(4):777-84. doi: 10.3892/ijo_00000553. Int J Oncol. 2010. PMID: 20198319
-
Alterations in microtubules, intermediate filaments, and microfilaments induced by microcystin-LR in cultured cells.Toxicol Pathol. 1995 May-Jun;23(3):326-37. doi: 10.1177/019262339502300309. Toxicol Pathol. 1995. PMID: 7659955
-
Cytoskeletal crosstalk: A focus on intermediate filaments.Curr Opin Cell Biol. 2024 Apr;87:102325. doi: 10.1016/j.ceb.2024.102325. Epub 2024 Feb 14. Curr Opin Cell Biol. 2024. PMID: 38359728 Review.
-
Joining actions: crosstalk between intermediate filaments and actin orchestrates cellular physical dynamics and signaling.Sci China Life Sci. 2019 Oct;62(10):1368-1374. doi: 10.1007/s11427-018-9488-1. Epub 2019 May 14. Sci China Life Sci. 2019. PMID: 31098891 Review.
Cited by
-
The use of advanced imaging in guiding the further investigation and treatment of primary prostate cancer.Cancer Imaging. 2022 Sep 3;22(1):45. doi: 10.1186/s40644-022-00481-3. Cancer Imaging. 2022. PMID: 36057766 Free PMC article. Review.
-
Tuning Pharmacokinetics to Improve Tumor Accumulation of a Prostate-Specific Membrane Antigen-Targeted Phototheranostic Agent.Bioconjug Chem. 2018 Nov 21;29(11):3746-3756. doi: 10.1021/acs.bioconjchem.8b00636. Epub 2018 Oct 29. Bioconjug Chem. 2018. PMID: 30350576 Free PMC article.
-
From AR to c-Met: androgen deprivation leads to a signaling pathway switch in prostate cancer cells.Int J Oncol. 2013 Oct;43(4):1125-30. doi: 10.3892/ijo.2013.2020. Epub 2013 Jul 18. Int J Oncol. 2013. PMID: 23877345 Free PMC article.
-
Targeting prostate cancer cells with PSMA inhibitor-guided gold nanoparticles.Bioorg Med Chem Lett. 2013 Jan 15;23(2):565-8. doi: 10.1016/j.bmcl.2012.11.015. Epub 2012 Nov 16. Bioorg Med Chem Lett. 2013. PMID: 23232055 Free PMC article.
-
Repurposing of PSMA-targeted diagnostic and therapeutic agents for the detection and treatment of giant cell tumors of bone.Front Oncol. 2024 Nov 15;14:1504514. doi: 10.3389/fonc.2024.1504514. eCollection 2024. Front Oncol. 2024. PMID: 39619440 Free PMC article.
References
-
- Allison RR, Downie GH, Cuenca R, Hu XH, Childs CJ, Sibata CH. Photosensitizers in clinical PDT. Photodiagnosis and Photodynamic Therapy. 2004;1:27–42. - PubMed
-
- Sharman WM, van Lier JE, Allen CM. Targeted photodynamic therapy via receptor mediated delivery systems. Adv Drug Deliv Rev. 2004;56:53–76. - PubMed
-
- Detty MR, Gibson SL, Wagner SJ. Current clinical and preclinical photosensitizers for use in photodynamic therapy. Journal of medicinal chemistry. 2004;47:3897–3915. - PubMed
-
- Berg K, Selbo PK, Weyergang A, Dietze A, Prasmickaite L, Bonsted A, Engesaeter BO, Angell-Petersen E, Warloe T, Frandsen N, Hogset A. Porphyrin-related photosensitizers for cancer imaging and therapeutic applications. J Microsc. 2005;218:133–147. - PubMed
-
- Moore CM, Pendse D, Emberton M. Photodynamic therapy for prostate cancer--a review of current status and future promise. Nature clinical practice. Urology. 2009;6:18–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

