Regenerative healing following foetal myocardial infarction
- PMID: 20452780
- PMCID: PMC2921018
- DOI: 10.1016/j.ejcts.2010.03.049
Regenerative healing following foetal myocardial infarction
Abstract
Objectives: The adult response to myocardial infarction results in inflammation, scar formation, left ventricular dilatation, and loss of regional and global function. Regenerative scarless healing has been demonstrated in foetal dermis and tendon and is associated with diminished inflammation. We hypothesised that following foetal myocardial infarction, there would be minimal inflammation, regenerative healing, and preservation of function.
Methods: Anteroapical myocardial infarction encompassing 20% of the left ventricle was created in adult or early gestation foetal sheep. Myocardial function was serially assessed using quantitative echocardiography. Infarct architecture was examined histologically for evidence of scar formation. Cellular inflammation, cellular proliferation, and apoptosis were assessed using immunohistochemistry.
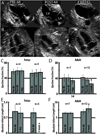
Results: In the adult sheep 4 weeks following myocardial infarction, there was a significant decline in ejection fraction (EF) (41±7.4% to 26±7.4%, p<0.05), and the akinetic myocardial segment increased in size (6.9±0.8 cm to 7.9±1.1 cm, p<0.05). By contrast, there was no decline in the foetal EF (53±8.1% to 55±8.8%) and no akinetic foetal myocardial segment 4 weeks post-infarction. The foetal infarcts lacked an inflammatory cell infiltrate and healed with minimal fibrosis, compared with the adults. Foetal infarcts also demonstrated 5-bromo-2'-deoxyuridine (BrdU)+ proliferating cells, including cardiomyocytes, within the infarct.
Conclusions: These data demonstrate that the foetal response to myocardial infarction is dramatically different from the adult and is characterised by minimal inflammation, lack of fibrosis, myocardial proliferation and restoration of cardiac function. Diminished inflammation is associated with foetal regenerative cardiac healing following injury. Understanding the mechanisms involved in foetal myocardial regeneration may lead to applications to alter the adult response following myocardial infarction.
Copyright © 2010 European Association for Cardio-Thoracic Surgery. Published by Elsevier B.V. All rights reserved.
Figures



References
-
- Yang Z, Day YJ, Toufektsian MC, Xu Y, Ramos SI, Marshall MA, French BA, Linden J. Myocardial infarct-sparing effect of adenosine A2A Receptor Activation is due to its action on CD4+ T lymphocytes. Circulation. 2006;114:2056–2064. - PubMed
-
- St. John Sutton M, Sharpe N. Left ventricular remodeling after infarction: pathophysiology and therapy. Circulation. 2000;101:2981–2988. - PubMed
-
- Cleutjens JPM, Kandala JC, Guarda E, Guntaka RV, Weber KT. Regulation of collagen degradation in the rat myocardium after infarction. J Mol Cell Cardiol. 1995;27:1281–1292. - PubMed
-
- Takahashi T, Hiasa Y, Ohara Y, Miyazaki S, Ogura R, Miyajima H, Yuba K, Suzuki N, Hosokawa S, Kishi K, Ohtani R. Relation between neutrophil counts on admission, microvascular injury, and left ventricular functional recovery in patients with an anterior wall first acute myocardial infarction treated with primary coronary angioplasty. Am J Cardiol. 2007;100:35–40. - PubMed
-
- Åströ-Olsson K, Hedström E, Hultén LM, Wiklund O, Arheden H, Öhlin AK, Gottsäter A, Öhlin H. Dissociation of the inflammatory reaction following PCI for acute myocardial infarction. J Invasive Cardiol. 2007;19:452–456. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

