Beyond alphabeta/gammadelta lineage commitment: TCR signal strength regulates gammadelta T cell maturation and effector fate
- PMID: 20452783
- PMCID: PMC3129014
- DOI: 10.1016/j.smim.2010.04.006
Beyond alphabeta/gammadelta lineage commitment: TCR signal strength regulates gammadelta T cell maturation and effector fate
Abstract
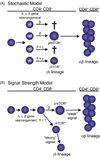
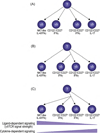
Signaling by the gammadelta T cell receptor (TCR) is required not only for alphabeta/gammadelta lineage commitment but also to activate and elicit effector functions in mature gammadelta T cells. Notably, at both of these stages, the signal delivered by the gammadeltaTCR is more robust than the one delivered by either the preTCR or the alphabetaTCR. Recent studies now provide evidence that signaling by the gammadeltaTCR is also required at other stages during gammadelta T cell development. Remarkably, the strength of the gammadeltaTCR signal also plays a role at these other stages, as evidenced by the findings that genetic manipulation of gammadeltaTCR signal strength affects gammadelta T cell maturation and effector fate. In this review, we discuss how a strong TCR signal is a recurring theme in gammadelta T cell development and activation.
2010 Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
αβ versus γδ fate choice: counting the T-cell lineages at the branch point.Immunol Rev. 2010 Nov;238(1):169-81. doi: 10.1111/j.1600-065X.2010.00947.x. Immunol Rev. 2010. PMID: 20969592 Free PMC article. Review.
-
Disorderly conduct in gammadelta versus alphabeta T cell lineage commitment.Semin Immunol. 2010 Aug;22(4):222-7. doi: 10.1016/j.smim.2010.04.003. Epub 2010 May 6. Semin Immunol. 2010. PMID: 20451409 Free PMC article. Review.
-
gammadeltaTCR ligands and lineage commitment.Semin Immunol. 2010 Aug;22(4):214-21. doi: 10.1016/j.smim.2010.04.001. Epub 2010 May 5. Semin Immunol. 2010. PMID: 20447836 Free PMC article. Review.
-
gammadelta and alphabeta T cell lineage choice: resolution by a stronger sense of being.Semin Immunol. 2010 Aug;22(4):228-36. doi: 10.1016/j.smim.2010.04.005. Epub 2010 May 13. Semin Immunol. 2010. PMID: 20466561 Review.
-
Towards a molecular understanding of the differential signals regulating alphabeta/gammadelta T lineage choice.Semin Immunol. 2010 Aug;22(4):237-46. doi: 10.1016/j.smim.2010.04.008. Epub 2010 May 14. Semin Immunol. 2010. PMID: 20471282 Free PMC article. Review.
Cited by
-
Shaping immunity for life: Layered development of CD8+ T cells.Immunol Rev. 2023 May;315(1):108-125. doi: 10.1111/imr.13185. Epub 2023 Jan 18. Immunol Rev. 2023. PMID: 36653953 Free PMC article. Review.
-
PreTCR and TCRγδ signal initiation in thymocyte progenitors does not require domains implicated in receptor oligomerization.Sci Signal. 2011 Jul 19;4(182):ra47. doi: 10.1126/scisignal.2001765. Sci Signal. 2011. PMID: 21775286 Free PMC article.
-
Selection of self-reactive T cells in the thymus.Annu Rev Immunol. 2012;30:95-114. doi: 10.1146/annurev-immunol-020711-075035. Epub 2011 Dec 5. Annu Rev Immunol. 2012. PMID: 22149933 Free PMC article. Review.
-
The gene regulatory basis of bystander activation in CD8+ T cells.Sci Immunol. 2024 Feb 23;9(92):eadf8776. doi: 10.1126/sciimmunol.adf8776. Epub 2024 Feb 23. Sci Immunol. 2024. PMID: 38394230 Free PMC article.
-
Targeting CD147 for T to NK Lineage Reprogramming and Tumor Therapy.EBioMedicine. 2017 Jun;20:98-108. doi: 10.1016/j.ebiom.2017.05.022. Epub 2017 May 18. EBioMedicine. 2017. PMID: 28571672 Free PMC article.
References
-
- Chien Y-H, Jores R, Crowley MP. Recognition by γ/δ T cells. Annu Rev Immunol. 1996;14:511–532. - PubMed
-
- Boismenu R, Havran WL. γδ T cells in host defense and epithelial cell biology. Clin Immunol Immunopathol. 1998;86:121–133. - PubMed
-
- Hayday AC. γδcells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. - PubMed
-
- Carding SR, Egan PJ. γδ T cells: functional plasticity and heterogeneity. Nat Rev Immunol. 2002;2:336–345. - PubMed
-
- Hayday A, Tigelaar R. Immunoregulation in the tissues by γδ T cells. Nat Rev Immunol. 2003;2:233–242. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

