Lamin-binding Proteins
- PMID: 20452940
- PMCID: PMC2845209
- DOI: 10.1101/cshperspect.a000554
Lamin-binding Proteins
Abstract
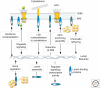
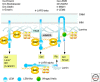
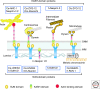
A- and B-type lamins are the major intermediate filaments of the nucleus. Lamins engage in a plethora of stable and transient interactions, near the inner nuclear membrane and throughout the nucleus. Lamin-binding proteins serve an amazingly diverse range of functions. Numerous inner-membrane proteins help anchor lamin filaments to the nuclear envelope, serving as part of the nuclear "lamina" network that is essential for nuclear architecture and integrity. Certain lamin-binding proteins of the inner membrane bind partners in the outer membrane and mechanically link lamins to the cytoskeleton. Inside the nucleus, lamin-binding proteins appear to serve as the "adaptors" by which the lamina organizes chromatin, influences gene expression and epigenetic regulation, and modulates signaling pathways. Transient interactions of lamins with key components of the transcription and replication machinery may provide an additional level of regulation or support to these essential events.
Figures
References
-
- Anderson DJ, Hetzer MW 2008. Shaping the endoplasmic reticulum into the nuclear envelope. J Cell Sci 121:137–142 - PubMed
-
- Bakay M, Wang Z, Melcon G, Schiltz L, Xuan J, Zhao P, Sartorelli V, Seo J, Pegoraro E, Angelini C, et al. 2006. Nuclear envelope dystrophies show a transcriptional fingerprint suggesting disruption of Rb-MyoD pathways in muscle regeneration. Brain 129:996–1013 - PubMed
-
- Bao X, Zhang W, Krencik R, Deng H, Wang Y, Girton J, Johansen J, Johansen KM 2005. The JIL-1 kinase interacts with lamin Dm0 and regulates nuclear lamina morphology of Drosophila nurse cells. J Cell Sci 118:5079–5087 - PubMed
-
- Barton RM, Worman HJ 1999. Prenylated prelamin A interacts with Narf, a novel nuclear protein. J Biol Chem 274:30008–30018 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
