Initiating and growing an axon
- PMID: 20452947
- PMCID: PMC2845204
- DOI: 10.1101/cshperspect.a001925
Initiating and growing an axon
Abstract
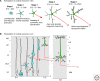
The ability of neurons to form a single axon and multiple dendrites underlies the directional flow of information transfer in the central nervous system. Dendrites and axons are molecularly and functionally distinct domains. Dendrites integrate synaptic inputs, triggering the generation of action potentials at the level of the soma. Action potentials then propagate along the axon, which makes presynaptic contacts onto target cells. This article reviews what is known about the cellular and molecular mechanisms underlying the ability of neurons to initiate and extend a single axon during development. Remarkably, neurons can polarize to form a single axon, multiple dendrites, and later establish functional synaptic contacts in reductionist in vitro conditions. This approach became, and remains, the dominant model to study axon initiation and growth and has yielded the identification of many molecules that regulate axon formation in vitro (Dotti et al. 1988). At present, only a few of the genes identified using in vitro approaches have been shown to be required for axon initiation and outgrowth in vivo. In vitro, axon initiation and elongation are largely intrinsic properties of neurons that are established in the absence of relevant extracellular cues. However, the importance of extracellular cues to axon initiation and outgrowth in vivo is emerging as a major theme in neural development (Barnes and Polleux 2009). In this article, we focus our attention on the extracellular cues and signaling pathways required in vivo for axon initiation and axon extension.
Figures
References
-
- Akhtar RS, Ness JM, Roth KA 2004. Bcl-2 family regulation of neuronal development and neurodegeneration. Biochim Biophys Acta 1644:189–203 - PubMed
-
- Aoki K, Taketo MM 2007. Adenomatous polyposis coli (APC): A multi-functional tumor suppressor gene. J Cell Sci 120:3327–3335 - PubMed
-
- Aravamudan B, Broadie K 2003. Synaptic Drosophila UNC-13 is regulated by antagonistic G-protein pathways via a proteasome-dependent degradation mechanism. J Neurobiol 54:417–438 - PubMed
-
- Arimura N, Kaibuchi K 2007. Neuronal polarity: From extracellular signals to intracellular mechanisms. Nat Rev Neurosci 8:194–205 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
