The origin of biological homochirality
- PMID: 20452962
- PMCID: PMC2857173
- DOI: 10.1101/cshperspect.a002147
The origin of biological homochirality
Abstract
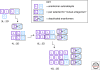
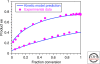
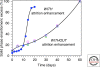
The single-handedness of biological molecules has fascinated scientists and laymen alike since Pasteur's first painstaking separation of the enantiomorphic crystals of a tartrate salt more than 150 yr ago. More recently, a number of theoretical and experimental investigations have helped to delineate models for how one enantiomer might have come to dominate over the other from what presumably was a racemic prebiotic world. This article highlights mechanisms for enantioenrichment that include either chemical or physical processes, or a combination of both. The scientific driving force for this work arises from an interest in understanding the origin of life, because the homochirality of biological molecules is a signature of life.
Figures

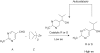








References
-
- Ball P 2007. Giving life a hand. Chem World 4:30–31
-
- Blackmond DG 2006. Mechanistic study of the Soai autocatalytic reaction informed by kinetic analysis. Tetrahedron: Asymmetry 17:584–589
-
- Blackmond DG 2009. The double solubility rule holds for racemizing enantiomers. Chem Eur J 15:3065–3068 - PubMed
-
- Blackmond DG, Klussmann M 2007a. Spoilt for choice: Assessing phase behavior models for the evolution of homochirality. Chem Commun 3990–3996 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
