Mineral surfaces, geochemical complexities, and the origins of life
- PMID: 20452963
- PMCID: PMC2857174
- DOI: 10.1101/cshperspect.a002162
Mineral surfaces, geochemical complexities, and the origins of life
Abstract
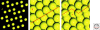
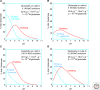
Crystalline surfaces of common rock-forming minerals are likely to have played several important roles in life's geochemical origins. Transition metal sulfides and oxides promote a variety of organic reactions, including nitrogen reduction, hydroformylation, amination, and Fischer-Tropsch-type synthesis. Fine-grained clay minerals and hydroxides facilitate lipid self-organization and condensation polymerization reactions, notably of RNA monomers. Surfaces of common rock-forming oxides, silicates, and carbonates select and concentrate specific amino acids, sugars, and other molecular species, while potentially enhancing their thermal stabilities. Chiral surfaces of these minerals also have been shown to separate left- and right-handed molecules. Thus, mineral surfaces may have contributed centrally to the linked prebiotic problems of containment and organization by promoting the transition from a dilute prebiotic "soup" to highly ordered local domains of key biomolecules.
Figures


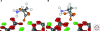
References
-
- Adam Z 2007. Actinides and life’s origins. Astrobiology 7:852–872 - PubMed
-
- Ahmadi A, Attard G, Feliu J, Rodes A 1999. Surface reactivity at “chiral” platinum surfaces. Langmuir 15:2420–2424
-
- Albarède F, Hoffmann AW 2003. Geochemistry: An introduction Cambridge University Press, Cambridge, UK
-
- Amariglio A, Amariglio H, Duval X 1968. Asymmetric reactions on optically active quartz. Helv Chim Acta 51:2110
-
- Arora AK, Kamaluddin 2009. Role of metal oxides in chemical evolution: Interaction of ribose nucleotide with alumina. Astrobiology 9:165–171 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
