Impaired de novo choline synthesis explains why phosphatidylethanolamine N-methyltransferase-deficient mice are protected from diet-induced obesity
- PMID: 20452975
- PMCID: PMC2903412
- DOI: 10.1074/jbc.M110.108514
Impaired de novo choline synthesis explains why phosphatidylethanolamine N-methyltransferase-deficient mice are protected from diet-induced obesity
Abstract
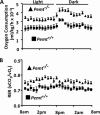
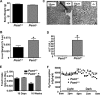
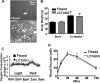
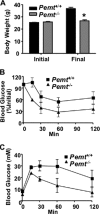
Phosphatidylcholine (PC) is synthesized from choline via the CDP-choline pathway. Liver cells can also synthesize PC via the sequential methylation of phosphatidylethanolamine, catalyzed by phosphatidylethanolamine N-methyltransferase (PEMT). The current study investigates whether or not hepatic PC biosynthesis is linked to diet-induced obesity. Pemt(+/+) mice fed a high fat diet for 10 weeks increased in body mass by 60% and displayed insulin resistance, whereas Pemt(-/-) mice did not. Compared with Pemt(+/+) mice, Pemt(-/-) mice had increased energy expenditure and maintained normal peripheral insulin sensitivity; however, they developed hepatomegaly and steatosis. In contrast, mice with impaired biosynthesis of PC via the CDP-choline pathway in liver became obese when fed a high fat diet. We, therefore, hypothesized that insufficient choline, rather than decreased hepatic phosphatidylcholine, was responsible for the lack of weight gain in Pemt(-/-) mice despite the presence of 1.3 g of choline/kg high fat diet. Supplementation with an additional 2.7 g of choline (but not betaine)/kg of diet normalized energy metabolism, weight gain, and insulin resistance in high fat diet-fed Pemt(-/-) mice. Furthermore, Pemt(+/+) mice that were fed a choline-deficient diet had increased oxygen consumption, had improved glucose tolerance, and gained less weight. Thus, de novo synthesis of choline via PEMT has a previously unappreciated role in regulating whole body energy metabolism.
Figures
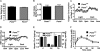



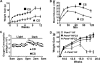
References
-
- Muoio D. M., Newgard C. B. (2006) Annu. Rev. Biochem. 75, 367–401 - PubMed
-
- Kalaany N. Y., Gauthier K. C., Zavacki A. M., Mammen P. P., Kitazume T., Peterson J. A., Horton J. D., Garry D. J., Bianco A. C., Mangelsdorf D. J. (2005) Cell Metab. 1, 231–244 - PubMed
-
- Hubbard B., Doege H., Punreddy S., Wu H., Huang X., Kaushik V. K., Mozell R. L., Byrnes J. J., Stricker-Krongrad A., Chou C. J., Tartaglia L. A., Lodish H. F., Stahl A., Gimeno R. E. (2006) Gastroenterology 130, 1259–1269 - PubMed
-
- Pospisilik J. A., Knauf C., Joza N., Benit P., Orthofer M., Cani P. D., Ebersberger I., Nakashima T., Sarao R., Neely G., Esterbauer H., Kozlov A., Kahn C. R., Kroemer G., Rustin P., Burcelin R., Penninger J. M. (2007) Cell 131, 476–491 - PubMed
-
- Uno K., Katagiri H., Yamada T., Ishigaki Y., Ogihara T., Imai J., Hasegawa Y., Gao J., Kaneko K., Iwasaki H., Ishihara H., Sasano H., Inukai K., Mizuguchi H., Asano T., Shiota M., Nakazato M., Oka Y. (2006) Science 312, 1656–1659 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

