The in vivo toxicity of hydroxyurea depends on its direct target catalase
- PMID: 20452979
- PMCID: PMC2898382
- DOI: 10.1074/jbc.M110.103564
The in vivo toxicity of hydroxyurea depends on its direct target catalase
Abstract
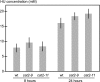
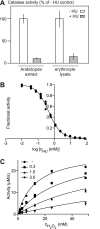
Hydroxyurea (HU) is a well tolerated ribonucleotide reductase inhibitor effective in HIV, sickle cell disease, and blood cancer therapy. Despite a positive initial response, however, most treated cancers eventually progress due to development of HU resistance. Although RNR properties influence HU resistance in cell lines, the mechanisms underlying cancer HU resistance in vivo remain unclear. To address this issue, we screened for HU resistance in the plant Arabidopsis thaliana and identified seventeen unique catalase mutants, thereby establishing that HU toxicity depends on catalase in vivo. We further demonstrated that catalase is a direct HU target by showing that HU acts as a competitive inhibitor of catalase-mediated hydrogen peroxide decomposition. Considering also that catalase can accelerate HU decomposition in vitro and that co-treatment with another catalase inhibitor alleviates HU effects in vivo, our findings suggests that HU could act as a catalase-activated pro-drug. Clinically, we found high catalase activity in circulating cells from untreated chronic myeloid leukemia, offering a possible explanation for the efficacy of HU against this malignancy.
Figures




References
-
- Navarra P., Preziosi P. (1999) Crit. Rev. Oncol. Hematol. 29, 249–255 - PubMed
-
- Lori F., Foli A., Kelly L. M., Lisziewicz J. (2007) Curr. Med. Chem. 14, 233–241 - PubMed
-
- Dalziel K., Round A., Stein K., Garside R., Price A. (2004) Health Technol. Assess. 8, iii, 1–120 - PubMed
-
- Elford H. L. (1968) Biochem. Biophys. Res. Commun. 33, 129–135 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

