Rapid decay of unstable Leishmania mRNAs bearing a conserved retroposon signature 3'-UTR motif is initiated by a site-specific endonucleolytic cleavage without prior deadenylation
- PMID: 20453029
- PMCID: PMC2943621
- DOI: 10.1093/nar/gkq349
Rapid decay of unstable Leishmania mRNAs bearing a conserved retroposon signature 3'-UTR motif is initiated by a site-specific endonucleolytic cleavage without prior deadenylation
Abstract
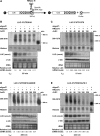
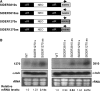
We have previously shown that the Leishmania genome possess two widespread families of extinct retroposons termed Short Interspersed DEgenerated Retroposons (SIDER1/2) that play a role in post-transcriptional regulation. Moreover, we have demonstrated that SIDER2 retroposons promote mRNA degradation. Here we provide new insights into the mechanism by which unstable Leishmania mRNAs harboring a SIDER2 retroposon in their 3'-untranslated region are degraded. We show that, unlike most eukaryotic transcripts, SIDER2-bearing mRNAs do not undergo poly(A) tail shortening prior to rapid turnover, but instead, they are targeted for degradation by a site-specific endonucleolytic cleavage. The main cleavage site was mapped in two randomly selected SIDER2-containing mRNAs in vivo between an AU dinucleotide at the 5'-end of the second 79-nt signature (signature II), which represents the most conserved sequence amongst SIDER2 retroposons. Deletion of signature II abolished endonucleolytic cleavage and deadenylation-independent decay and increased mRNA stability. Interestingly, we show that overexpression of SIDER2 anti-sense RNA can increase sense transcript abundance and stability, and that complementarity to the cleavage region is required for protecting SIDER2-containing transcripts from degradation. These results establish a new paradigm for how unstable mRNAs are degraded in Leishmania and could serve as the basis for a better understanding of mRNA decay pathways in general.
Figures



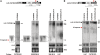
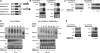
Similar articles
-
RNA secondary structure and nucleotide composition of the conserved hallmark sequence of Leishmania SIDER2 retroposons are essential for endonucleolytic cleavage and mRNA degradation.PLoS One. 2017 Jul 13;12(7):e0180678. doi: 10.1371/journal.pone.0180678. eCollection 2017. PLoS One. 2017. PMID: 28704426 Free PMC article.
-
The Pumilio-domain protein PUF6 contributes to SIDER2 retroposon-mediated mRNA decay in Leishmania.RNA. 2017 Dec;23(12):1874-1885. doi: 10.1261/rna.062950.117. Epub 2017 Sep 6. RNA. 2017. PMID: 28877997 Free PMC article.
-
Approaches for studying mRNA decay mediated by SIDER2 retroposons in Leishmania.Methods Mol Biol. 2015;1201:123-42. doi: 10.1007/978-1-4939-1438-8_7. Methods Mol Biol. 2015. PMID: 25388111
-
An erythroid-enriched endoribonuclease (ErEN) involved in alpha-globin mRNA turnover.Protein Pept Lett. 2007;14(2):131-6. doi: 10.2174/092986607779816168. Protein Pept Lett. 2007. PMID: 17305599 Review.
-
Implications of polyadenylation in health and disease.Nucleus. 2014;5(6):508-19. doi: 10.4161/nucl.36360. Epub 2014 Oct 31. Nucleus. 2014. PMID: 25484187 Free PMC article. Review.
Cited by
-
The structure and repertoire of small interfering RNAs in Leishmania (Viannia) braziliensis reveal diversification in the trypanosomatid RNAi pathway.Mol Microbiol. 2013 Feb;87(3):580-93. doi: 10.1111/mmi.12117. Epub 2012 Dec 26. Mol Microbiol. 2013. PMID: 23217017 Free PMC article.
-
RNA secondary structure and nucleotide composition of the conserved hallmark sequence of Leishmania SIDER2 retroposons are essential for endonucleolytic cleavage and mRNA degradation.PLoS One. 2017 Jul 13;12(7):e0180678. doi: 10.1371/journal.pone.0180678. eCollection 2017. PLoS One. 2017. PMID: 28704426 Free PMC article.
-
Evolutionary divergent clusters of transcribed extinct truncated retroposons drive low mRNA expression and developmental regulation in the protozoan Leishmania.BMC Biol. 2024 Oct 29;22(1):249. doi: 10.1186/s12915-024-02051-4. BMC Biol. 2024. PMID: 39468514 Free PMC article.
-
The roles of 3'-exoribonucleases and the exosome in trypanosome mRNA degradation.RNA. 2013 Jul;19(7):937-47. doi: 10.1261/rna.038430.113. Epub 2013 May 22. RNA. 2013. PMID: 23697549 Free PMC article.
-
The Pumilio-domain protein PUF6 contributes to SIDER2 retroposon-mediated mRNA decay in Leishmania.RNA. 2017 Dec;23(12):1874-1885. doi: 10.1261/rna.062950.117. Epub 2017 Sep 6. RNA. 2017. PMID: 28877997 Free PMC article.
References
-
- Martinez-Calvillo S, Yan S, Nguyen D, Fox M, Stuart K, Myler PJ. Transcription of Leishmania major Friedlin chromosome 1 initiates in both directions within a single region. Mol. Cell. 2003;11:1291–1299. - PubMed
-
- Clayton C, Shapira M. Post-transcriptional regulation of gene expression in trypanosomes and leishmanias. Mol. Biochem. Parasitol. 2007;156:93–101. - PubMed
-
- Haile S, Papadopoulou B. Developmental regulation of gene expression in trypanosomatid parasitic protozoa. Curr. Opin. Microbiol. 2007;10:569–577. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

