Aldosterone and vasopressin affect {alpha}- and {gamma}-ENaC mRNA translation
- PMID: 20453031
- PMCID: PMC2943617
- DOI: 10.1093/nar/gkq267
Aldosterone and vasopressin affect {alpha}- and {gamma}-ENaC mRNA translation
Abstract
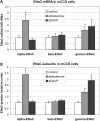
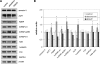
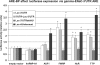
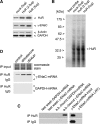
Vasopressin and aldosterone play key roles in the fine adjustment of sodium and water re-absorption in the nephron. The molecular target of this regulation is the epithelial sodium channel (ENaC) consisting of α-, β- and γ-subunits. We investigated mRNA-specific post-transcriptional mechanisms in hormone-dependent expression of ENaC subunits in mouse kidney cortical collecting duct cells. Transcription experiments and polysome gradient analysis demonstrate that both hormones act on transcription and translation. RNA-binding proteins (RBPs) and mRNA sequence motifs involved in translational control of γ-ENaC synthesis were studied. γ-ENaC-mRNA 3'-UTR contains an AU-rich element (ARE), which was shown by RNA affinity chromatography to interact with AU-rich element binding proteins (ARE-BP) like HuR, AUF1 and TTP. Some RBPs co-localized with γ-ENaC mRNA in polysomes in a hormone-dependent manner. Reporter gene co-expression experiments with luciferase γ-ENaC 3'-UTR constructs and ARE-BP expression plasmids demonstrate the importance of RNA-protein interaction for the up-regulation of γ-ENaC synthesis. We document that aldosterone and the V(2) receptor agonist dDAVP act on synthesis of α- and γ-ENaC subunits mediated by RBPs as effectors of translation but not by mRNA stabilization. Immunoprecipitation and UV-crosslinking analysis of γ-ENaC-mRNA/HuR complexes document the significance of γ-ENaC-mRNA-3'-UTR/HuR interaction for hormonal control of ENaC synthesis.
Figures






Similar articles
-
Chronic exposure to vasopressin upregulates ENaC and sodium transport in the rat renal collecting duct and lung.Hypertension. 2001 Nov;38(5):1143-9. doi: 10.1161/hy1001.092641. Hypertension. 2001. PMID: 11711512
-
Interaction between 3' untranslated region of calcitonin receptor messenger ribonucleic acid (RNA) and adenylate/uridylate (AU)-rich element binding proteins (AU-rich RNA-binding factor 1 and Hu antigen R).Endocrinology. 2004 Apr;145(4):1730-8. doi: 10.1210/en.2003-0862. Epub 2004 Jan 8. Endocrinology. 2004. PMID: 14715706
-
Sirtuin 1 functionally and physically interacts with disruptor of telomeric silencing-1 to regulate alpha-ENaC transcription in collecting duct.J Biol Chem. 2009 Jul 31;284(31):20917-26. doi: 10.1074/jbc.M109.020073. Epub 2009 Jun 2. J Biol Chem. 2009. PMID: 19491102 Free PMC article.
-
[Regulation by vasopressin of NaCl absorption in the renal collecting duct].J Soc Biol. 2005;199(4):361-8. doi: 10.1051/jbio:2005038. J Soc Biol. 2005. PMID: 16738531 Review. French.
-
Epigenetics and the control of the collecting duct epithelial sodium channel.Semin Nephrol. 2013 Jul;33(4):383-91. doi: 10.1016/j.semnephrol.2013.05.010. Semin Nephrol. 2013. PMID: 24011580 Free PMC article. Review.
Cited by
-
NHA2 promotes cyst development in an in vitro model of polycystic kidney disease.J Physiol. 2019 Jan;597(2):499-519. doi: 10.1113/JP276796. Epub 2018 Oct 17. J Physiol. 2019. PMID: 30242840 Free PMC article.
-
Nav1.9 channel contributes to mechanical and heat pain hypersensitivity induced by subacute and chronic inflammation.PLoS One. 2011;6(8):e23083. doi: 10.1371/journal.pone.0023083. Epub 2011 Aug 12. PLoS One. 2011. PMID: 21857998 Free PMC article.
-
Landscape of GPCR expression along the mouse nephron.Am J Physiol Renal Physiol. 2021 Jul 1;321(1):F50-F68. doi: 10.1152/ajprenal.00077.2021. Epub 2021 May 24. Am J Physiol Renal Physiol. 2021. PMID: 34029142 Free PMC article.
-
Annexin II Light Chain p11 Interacts With ENaC to Increase Functional Activity at the Membrane.Front Physiol. 2019 Feb 8;10:7. doi: 10.3389/fphys.2019.00007. eCollection 2019. Front Physiol. 2019. PMID: 30800070 Free PMC article.
-
Altered ENaC is Associated With Aortic Baroreceptor Dysfunction in Chronic Heart Failure.Am J Hypertens. 2016 May;29(5):582-9. doi: 10.1093/ajh/hpv141. Epub 2015 Aug 20. Am J Hypertens. 2016. PMID: 26297031 Free PMC article.
References
-
- Alvarez de la Rosa DA, Canessa CM, Fyfe GK, Zhang P. Structure and regulation of amiloride-sensitive sodium channels. Ann. Rev. Physiol. 2000;62:573–594. - PubMed
-
- Rossier BC, Pradervand S, Schild L, Hummler E. Epithelial sodium channel and the control of sodium balance: interaction between genetic and invironmental factors. Ann. Rev. Physiol. 2002;64:877–897. - PubMed
-
- Snyder PM. The epithelial Na+ Channel: Cell Surface Insertion and Retrieval in Na+ Homeostasis and Hypertension. Endocr. Rev. 2002;23:258–275. - PubMed
-
- Weisz OA, Johnson JP. Noncoordinate regulation of ENaC: paradigm lost? Am. J. Physiol. Renal Physiol. 2003;285:F833–F842. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

