Prophase I arrest and progression to metaphase I in mouse oocytes: comparison of resumption of meiosis and recovery from G2-arrest in somatic cells
- PMID: 20453035
- PMCID: PMC2930517
- DOI: 10.1093/molehr/gaq034
Prophase I arrest and progression to metaphase I in mouse oocytes: comparison of resumption of meiosis and recovery from G2-arrest in somatic cells
Abstract
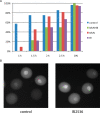
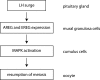
Mammalian oocytes are arrested at prophase I until puberty when luteinizing hormone (LH) induces resumption of meiosis of follicle-enclosed oocytes. Resumption of meiosis is tightly coupled with regulating cyclin-dependent kinase 1 (CDK1) activity. Prophase I arrest depends on inhibitory phosphorylation of CDK1 and anaphase-promoting complex-(APC-CDH1)-mediated regulation of cyclin B levels. Prophase I arrest is maintained by endogenously produced cyclic adenosine monophosphate (cAMP), which activates protein kinase A (PKA) that in turn phosphorylates (and activates) the nuclear kinase WEE2. In addition, PKA-mediated phosphorylation of the phosphatase CDC25B results in its cytoplasmic retention. The combined effect maintains low levels of CDK1 activity that are not sufficient to initiate resumption of meiosis. LH triggers synthesis of epidermal growth factor-like factors in mural granulosa cells and leads to reduced cGMP transfer from cumulus cells to oocytes via gap junctions that couple the two cell types. cGMP inhibits oocyte phosphodiesterase 3A (PDE3A) and a decline in oocyte cGMP results in increased PDE3A activity. The ensuing decrease in oocyte cAMP triggers maturation by alleviating the aforementioned phosphorylations of WEE2 and CDC25B. As a direct consequence CDC25B translocates into the nucleus. The resulting activation of CDK1 also promotes extrusion of WEE2 from the nucleus thereby providing a positive amplification mechanism for CDK1 activation. Other kinases, e.g. protein kinase B, Aurora kinase A and polo-like kinase 1, also participate in resumption of meiosis. Mechanisms governing meiotic prophase I arrest and resumption of meiosis share common features with DNA damage-induced mitotic G2-checkpoint arrest and checkpoint recovery, respectively. These common features include CDC14B-dependent activation of APC-CDH1 in prophase I arrested oocytes or G2-arrested somatic cells, and CDC25B-dependent cell cycle resumption in both oocytes and somatic cells.
Figures
References
-
- Alexandre H, Van Cauwenberge A, Tsukitani Y, Mulnard J. Pleiotropic effect of okadaic acid on maturing mouse oocytes. Development. 1991;112:971–980. - PubMed
-
- Barlow C, Liyanage M, Moens PB, Tarsounas M, Nagashima K, Brown K, Rottinghaus S, Jackson SP, Tagle D, Ried T, et al. Atm deficiency results in severe meiotic disruption as early as leptonema of prophase I. Development. 1998;125:4007–4017. - PubMed
-
- Bartek J, Lukas J. DNA damage checkpoints: from initiation to recovery or adaptation. Curr Opin Cell Biol. 2007;19:238–245. - PubMed
-
- Bornslaeger EA, Mattei P, Schultz RM. Involvement of cAMP-dependent protein kinase and protein phosphorylation in regulation of mouse oocyte maturation. Dev Biol. 1986;114:453–462. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

