Aspergillus fumigatus catalytic glucokinase and hexokinase: expression analysis and importance for germination, growth, and conidiation
- PMID: 20453072
- PMCID: PMC2901669
- DOI: 10.1128/EC.00362-09
Aspergillus fumigatus catalytic glucokinase and hexokinase: expression analysis and importance for germination, growth, and conidiation
Abstract
Fungi contain several hexokinases, which are involved either in sugar phosphorylation or in carbon source sensing. Glucose and fructose phosphorylations appear to rely exclusively on glucokinase and hexokinase. Here, we characterized the catalytic glucokinase and hexokinase from the opportunistic human pathogen Aspergillus fumigatus and showed that both enzymes display different biochemical properties and play different roles during growth and development. Glucokinase efficiently activates glucose and mannose but activates fructose only to a minor extent. Hexokinase showed a high efficiency for fructose activation but also activated glucose and mannose. Transcript and activity determinations revealed high levels of glucokinase in resting conidia, whereas hexokinase was associated mainly with the mycelium. Consequentially, a glucokinase mutant showed delayed germination at low glucose concentrations, whereas colony growth was not overly affected. The deletion of hexokinase had only a minor impact on germination but reduced colony growth, especially on sugar-containing media. Transcript determinations from infected mouse lungs revealed the expression of both genes, indicating a contribution to virulence. Interestingly, a double-deletion mutant showed impaired growth not only on sugars but also on nonfermentable nutrients, and growth on gluconeogenic carbon sources was strongly suppressed in the presence of glucose. Furthermore, the glkA hxkA deletion affected cell wall integrity, implying that both enzymes contribute to the cell wall composition. Additionally, the absence of either enzyme deregulated carbon catabolite repression since mutants displayed an induction of isocitrate lyase activity during growth on glucose-ethanol medium. Therefore, both enzymes seem to be required for balancing carbon flux in A. fumigatus and are indispensable for growth under all nutritional conditions.
Figures



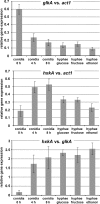


References
-
- Agger T., Petersen J. B., O'Connor S. M., Murphy R. L., Kelly J. M., Nielsen J. 2002. Physiological characterisation of recombinant Aspergillus nidulans strains with different creA genotypes expressing A. oryzae alpha-amylase. J. Biotechnol. 92:279–285 - PubMed
-
- Balloy V., Chignard M. 2009. The innate immune response to Aspergillus fumigatus. Microbes Infect. 11:919–927 - PubMed
-
- Bernardo S. M., Gray K. A., Todd R. B., Cheetham B. F., Katz M. E. 2007. Characterization of regulatory non-catalytic hexokinases in Aspergillus nidulans. Mol. Genet. Genomics 277:519–532 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

