Enhanced LH action in transgenic female mice expressing hCGbeta-subunit induces pituitary prolactinomas; the role of high progesterone levels
- PMID: 20453081
- PMCID: PMC2881531
- DOI: 10.1677/ERC-10-0016
Enhanced LH action in transgenic female mice expressing hCGbeta-subunit induces pituitary prolactinomas; the role of high progesterone levels
Abstract
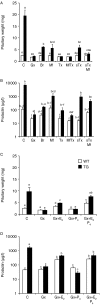
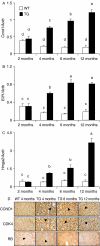
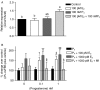
The etiology of pituitary adenomas remains largely unknown, with the exception of involvement of estrogens in the formation of prolactinomas. We have examined the molecular pathogenesis of prolactin-producing pituitary adenomas in transgenic female mice expressing the human choriongonadotropin (hCG) beta-subunit. The LH/CG bioactivity is elevated in the mice, with consequent highly stimulated ovarian progesterone (P(4)) production, in the face of normal estrogen secretion. Curiously, despite normal estrogen levels, large prolactinomas developed in these mice, and we provide here several lines of evidence that the elevated P(4) levels are involved in the growth of these estrogen-dependent tumors. The antiprogestin mifepristone inhibited tumor growth, and combined postgonadectomy estradiol/P(4) treatment was more effective than estrogen alone in inducing tumor growth. Evidence for direct growth-promoting effect of P(4) was obtained from cultures of primary mouse pituitary cells and rat somatomammotroph GH3 cells. The mouse tumors and cultured cells revealed stimulation of the cyclin D1/cyclin-dependent kinase 4/retinoblastoma protein/transcription factor E2F1 pathway in the growth response to P(4). If extrapolated to humans, and given the importance of endogenous P(4) and synthetic progestins in female reproductive functions and their pharmacotherapy, it is relevant to revisit the potential role of these hormones in the origin and growth of prolactinomas.
Figures

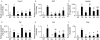
References
-
- Ahtiainen P, Rulli SB, Shariatmadari R, Pelliniemi LJ, Toppari J, Poutanen M, Huhtaniemi IT. Fetal but not adult Leydig cells are susceptible to adenoma formation in response to persistently high hCG level: a study on hCG overexpressing transgenic mice. Oncogene. 2005;24:7301–7309. - PubMed
-
- Bergendahl M, Perheentupa A, Huhtaniemi I. Effect of short-term starvation on reproductive hormone gene expression, secretion and receptor levels in male rats. Journal of Endocrinology. 1989;121:409–417. - PubMed
-
- Chen CL, Meites J. Effects of estrogen and progesterone on serum and pituitary prolactin levels in ovariectomized rats. Endocrinology. 1970;86:503–505. - PubMed
-
- Cicatiello L, Addeo R, Sasso A, Altucci L, Petrizzi VB, Borgo R, Cancemi M, Caporali S, Caristi S, Scafoglio C, et al. Estrogens and progesterone promote persistent CCND1 gene activation during G1 by inducing transcriptional derepression via c-Jun/c-Fos/estrogen receptor (progesterone receptor) complex assembly to a distal regulatory element and recruitment of cyclin D1 to its own gene promoter. Molecular and Cellular Biology. 2004;24:7260–7274. - PMC - PubMed
-
- Daly AF, Rixhon M, Adam C, Dempegioti A, Tichomirowa MA, Beckers A. High prevalence of pituitary adenomas: a cross-sectional study in the province of Liege, Belgium. Journal of Clinical Endocrinology and Metabolism. 2006;91:4769–4775. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

