ExsA recruits RNA polymerase to an extended -10 promoter by contacting region 4.2 of sigma-70
- PMID: 20453093
- PMCID: PMC2897327
- DOI: 10.1128/JB.00129-10
ExsA recruits RNA polymerase to an extended -10 promoter by contacting region 4.2 of sigma-70
Abstract
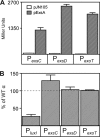
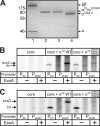
ExsA is a member of the AraC family of transcriptional activators and is required for expression of the Pseudomonas aeruginosa type III secretion system (T3SS). ExsA-dependent promoters consist of two binding sites for monomeric ExsA located approximately 50 bp upstream of the transcription start sites. Binding to both sites is required for recruitment of sigma(70)-RNA polymerase (RNAP) to the promoter. ExsA-dependent promoters also contain putative -35 hexamers that closely match the sigma(70) consensus but are atypically spaced 21 or 22 bp from the -10 hexamer. Because several nucleotides located within the putative -35 region are required for ExsA binding, it is unclear whether the putative -35 region makes an additional contribution to transcription initiation. In the present study we demonstrate that the putative -35 hexamer is dispensable for ExsA-independent transcription from the P(exsC) promoter and that deletion of sigma(70) region 4.2, which contacts the -35 hexamer, has no effect on ExsA-independent transcription from P(exsC). Region 4.2 of sigma(70), however, is required for ExsA-dependent activation of the P(exsC) and P(exsD) promoters. Genetic data suggest that ExsA directly contacts region 4.2 of sigma(70), and several amino acids were found to contribute to the interaction. In vitro transcription assays demonstrate that an extended -10 element located in the P(exsC) promoter is important for overall promoter activity. Our collective data suggest a model in which ExsA compensates for the lack of a -35 hexamer by interacting with region 4.2 of sigma(70) to recruit RNAP to the promoter.
Figures




Similar articles
-
Mechanism of transcriptional activation by Pseudomonas aeruginosa ExsA.J Bacteriol. 2009 Nov;191(21):6654-64. doi: 10.1128/JB.00902-09. Epub 2009 Aug 28. J Bacteriol. 2009. PMID: 19717612 Free PMC article.
-
ExsD inhibits expression of the Pseudomonas aeruginosa type III secretion system by disrupting ExsA self-association and DNA binding activity.J Bacteriol. 2010 Mar;192(6):1479-86. doi: 10.1128/JB.01457-09. Epub 2009 Dec 11. J Bacteriol. 2010. PMID: 20008065 Free PMC article.
-
Orientation of Pseudomonas aeruginosa ExsA monomers bound to promoter DNA and base-specific contacts with the P(exoT) promoter.J Bacteriol. 2012 May;194(10):2573-85. doi: 10.1128/JB.00107-12. Epub 2012 Mar 9. J Bacteriol. 2012. PMID: 22408167 Free PMC article.
-
Sigma and RNA polymerase: an on-again, off-again relationship?Mol Cell. 2005 Nov 11;20(3):335-45. doi: 10.1016/j.molcel.2005.10.015. Mol Cell. 2005. PMID: 16285916 Review.
-
Transcription activation in bacteria: ancient and modern.Microbiology (Reading). 2019 Apr;165(4):386-395. doi: 10.1099/mic.0.000783. Epub 2019 Feb 18. Microbiology (Reading). 2019. PMID: 30775965 Review.
Cited by
-
Regulation of bacterial virulence by Csr (Rsm) systems.Microbiol Mol Biol Rev. 2015 Jun;79(2):193-224. doi: 10.1128/MMBR.00052-14. Microbiol Mol Biol Rev. 2015. PMID: 25833324 Free PMC article. Review.
-
Backbone Interactions Between Transcriptional Activator ExsA and Anti-Activator ExsD Facilitate Regulation of the Type III Secretion System in Pseudomonas aeruginosa.Sci Rep. 2020 Jun 18;10(1):9881. doi: 10.1038/s41598-020-66555-z. Sci Rep. 2020. PMID: 32555263 Free PMC article.
-
ExsA and LcrF recognize similar consensus binding sites, but differences in their oligomeric state influence interactions with promoter DNA.J Bacteriol. 2013 Dec;195(24):5639-50. doi: 10.1128/JB.00990-13. Epub 2013 Oct 18. J Bacteriol. 2013. PMID: 24142246 Free PMC article.
-
Structural Analysis of the Regulatory Domain of ExsA, a Key Transcriptional Regulator of the Type Three Secretion System in Pseudomonas aeruginosa.PLoS One. 2015 Aug 28;10(8):e0136533. doi: 10.1371/journal.pone.0136533. eCollection 2015. PLoS One. 2015. PMID: 26317977 Free PMC article.
-
Pseudomonas aeruginosa Magnesium Transporter MgtE Inhibits Type III Secretion System Gene Expression by Stimulating rsmYZ Transcription.J Bacteriol. 2017 Oct 31;199(23):e00268-17. doi: 10.1128/JB.00268-17. Print 2017 Dec 1. J Bacteriol. 2017. PMID: 28847924 Free PMC article.
References
-
- Barbieri, J. T., and J. Sun. 2004. Pseudomonas aeruginosa ExoS and ExoT. Rev. Physiol. Biochem. Pharmacol. 152:79-92. - PubMed
-
- Becher, A., and H. P. Schweizer. 2000. Integration-proficient Pseudomonas aeruginosa vectors for isolation of single-copy chromosomal lacZ and lux gene fusions. Biotechniques 29:948-950, 952. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

