Enhancement of survival and electricity production in an engineered bacterium by light-driven proton pumping
- PMID: 20453141
- PMCID: PMC2897463
- DOI: 10.1128/AEM.02425-09
Enhancement of survival and electricity production in an engineered bacterium by light-driven proton pumping
Abstract
Microorganisms can use complex photosystems or light-dependent proton pumps to generate membrane potential and/or reduce electron carriers to support growth. The discovery that proteorhodopsin is a light-dependent proton pump that can be expressed readily in recombinant bacteria enables development of new strategies to probe microbial physiology and to engineer microbes with new light-driven properties. Here, we describe functional expression of proteorhodopsin and light-induced changes in membrane potential in the bacterium Shewanella oneidensis strain MR-1. We report that there were significant increases in electrical current generation during illumination of electrochemical chambers containing S. oneidensis expressing proteorhodopsin. We present evidence that an engineered strain is able to consume lactate at an increased rate when it is illuminated, which is consistent with the hypothesis that proteorhodopsin activity enhances lactate uptake by increasing the proton motive force. Our results demonstrate that there is coupling of a light-driven process to electricity generation in a nonphotosynthetic engineered bacterium. Expression of proteorhodopsin also preserved the viability of the bacterium under nutrient-limited conditions, providing evidence that fulfillment of basic energy needs of organisms may explain the widespread distribution of proteorhodopsin in marine environments.
Figures

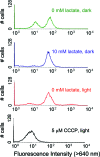



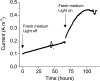

Similar articles
-
Proteorhodopsin Overproduction Enhances the Long-Term Viability of Escherichia coli.Appl Environ Microbiol. 2019 Dec 13;86(1):e02087-19. doi: 10.1128/AEM.02087-19. Print 2019 Dec 13. Appl Environ Microbiol. 2019. PMID: 31653788 Free PMC article.
-
Improved production of biohydrogen in light-powered Escherichia coli by co-expression of proteorhodopsin and heterologous hydrogenase.Microb Cell Fact. 2012 Jan 4;11:2. doi: 10.1186/1475-2859-11-2. Microb Cell Fact. 2012. PMID: 22217184 Free PMC article.
-
Proteorhodopsin is a light-driven proton pump with variable vectoriality.J Mol Biol. 2002 Aug 30;321(5):821-38. doi: 10.1016/s0022-2836(02)00696-4. J Mol Biol. 2002. PMID: 12206764
-
Potential of proton-pumping rhodopsins: engineering photosystems into microorganisms.Trends Biotechnol. 2013 Nov;31(11):633-42. doi: 10.1016/j.tibtech.2013.08.006. Epub 2013 Oct 9. Trends Biotechnol. 2013. PMID: 24120288 Review.
-
Marine Bacterial and Archaeal Ion-Pumping Rhodopsins: Genetic Diversity, Physiology, and Ecology.Microbiol Mol Biol Rev. 2016 Sep 14;80(4):929-54. doi: 10.1128/MMBR.00003-16. Print 2016 Dec. Microbiol Mol Biol Rev. 2016. PMID: 27630250 Free PMC article. Review.
Cited by
-
Engineering bionanoreactor in bacteria for efficient hydrogen production.Proc Natl Acad Sci U S A. 2024 Jul 16;121(29):e2404958121. doi: 10.1073/pnas.2404958121. Epub 2024 Jul 10. Proc Natl Acad Sci U S A. 2024. PMID: 38985767 Free PMC article.
-
Genetic Engineering of Microorganisms with Electroactive Genes for the Fabrication of Electrochemical Microbial Biosensors.Methods Mol Biol. 2024;2844:247-260. doi: 10.1007/978-1-0716-4063-0_17. Methods Mol Biol. 2024. PMID: 39068345
-
Shewanella oneidensis MR-1 Utilizes both Sodium- and Proton-Pumping NADH Dehydrogenases during Aerobic Growth.Appl Environ Microbiol. 2018 May 31;84(12):e00415-18. doi: 10.1128/AEM.00415-18. Print 2018 Jun 15. Appl Environ Microbiol. 2018. PMID: 29654176 Free PMC article.
-
Energy starved Candidatus Pelagibacter ubique substitutes light-mediated ATP production for endogenous carbon respiration.PLoS One. 2011 May 9;6(5):e19725. doi: 10.1371/journal.pone.0019725. PLoS One. 2011. PMID: 21573025 Free PMC article.
-
Design and development of synthetic microbial platform cells for bioenergy.Front Microbiol. 2013 Apr 19;4:92. doi: 10.3389/fmicb.2013.00092. eCollection 2013. Front Microbiol. 2013. PMID: 23626588 Free PMC article.
References
-
- Beja, O., L. Aravind, E. V. Koonin, M. T. Suzuki, A. Hadd, L. P. Nguyen, S. Jovanovich, C. M. Gates, R. A. Feldman, J. L. Spudich, E. N. Spudich, and E. F. DeLong. 2000. Bacterial rhodopsin: evidence for a new type of phototrophy in the sea. Science 289:1902-1906. - PubMed
-
- Beja, O., E. N. Spudich, J. L. Spudich, M. Leclerc, and E. F. DeLong. 2001. Proteorhodopsin phototrophy in the ocean. Nature 411:786-789. - PubMed
-
- Beliaev, A. S., D. A. Saffarini, J. L. McLaughlin, and D. Hunnicutt. 2001. MtrC, an outer membrane decahaem c cytochrome required for metal reduction in Shewanella putrefaciens MR-1. Mol. Microbiol. 39:722-730. - PubMed
-
- Burstein, C., L. Tiankova, and A. Kepes. 1979. Respiratory control in Escherichia coli K 12. Eur. J. Biochem. 94:387-392. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

