Nitric oxide synthase 3 contributes to ventilator-induced lung injury
- PMID: 20453164
- PMCID: PMC2928605
- DOI: 10.1152/ajplung.00341.2009
Nitric oxide synthase 3 contributes to ventilator-induced lung injury
Abstract
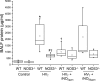
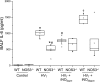
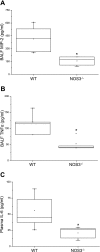
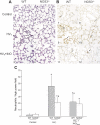
Nitric oxide synthase (NOS) depletion or inhibition reduces ventilator-induced lung injury (VILI), but the responsible mechanisms remain incompletely defined. The aim of this study was to elucidate the role of endothelial NOS, NOS3, in the pathogenesis of VILI in an in vivo mouse model. Wild-type and NOS3-deficient mice were ventilated with high-tidal volume (HV(T); 40 ml/kg) for 4 h, with and without adding NO to the inhaled gas. Additional wild-type mice were pretreated with tetrahydrobiopterin and ascorbic acid, agents that can prevent NOS-generated superoxide production. Arterial blood gas tensions, histology, and lung mechanics were evaluated after 4 h of HV(T) ventilation. The concentration of protein, IgM, cytokines, malondialdehyde, and 8-isoprostane were measured in bronchoalveolar lavage fluid (BALF). Myeloperoxidase activity, total and oxidized glutathione levels, and NOS-derived superoxide production were measured in lung tissue homogenates. HV(T) ventilation induced VILI in wild-type mice, as reflected by decreased lung compliance, increased concentrations of protein and cytokines in BALF, and oxidative stress. All indices of VILI were ameliorated in NOS3-deficient mice. Augmenting pulmonary NO levels by breathing NO during mechanical ventilation did not increase lung injury in NOS3-deficient mice. HV(T) ventilation increased NOS-inhibitable superoxide production in lung extracts from wild-type mice but not in those from NOS3-deficient mice. Administration of tetrahydrobiopterin and ascorbic acid ameliorated VILI in wild-type mice. Our results indicate that NOS3 contributes to ventilator-induced lung injury via increased production of superoxide.
Figures





Comment in
-
Understanding the role of NOS-3 in ventilator-induced lung injury: don't take NO for an answer.Am J Physiol Lung Cell Mol Physiol. 2010 Aug;299(2):L147-9. doi: 10.1152/ajplung.00179.2010. Epub 2010 Jun 4. Am J Physiol Lung Cell Mol Physiol. 2010. PMID: 20525916 Free PMC article. No abstract available.
References
-
- Abdulnour RE, Peng X, Finigan JH, Han EJ, Hasan EJ, Birukov KG, Reddy SP, Watkins JE, 3rd, Kayyali US, Garcia JG, Tuder RM, Hassoun PM. Mechanical stress activates xanthine oxidoreductase through MAP kinase-dependent pathways. Am J Physiol Lung Cell Mol Physiol 291: L345–L353, 2006 - PubMed
-
- Altemeier WA, Matute-Bello G, Gharib SA, Glenny RW, Martin TR, Liles WC. Modulation of lipopolysaccharide-induced gene transcription and promotion of lung injury by mechanical ventilation. J Immunol 175: 3369–3376, 2005 - PubMed
-
- Antoniades C, Shirodaria C, Warrick N, Cai S, de Bono J, Lee J, Leeson P, Neubauer S, Ratnatunga C, Pillai R, Refsum H, Channon KM. 5-Methyltetrahydrofolate rapidly improves endothelial function and decreases superoxide production in human vessels: effects on vascular tetrahydrobiopterin availability and endothelial nitric oxide synthase coupling. Circulation 114: 1193–1201, 2006 - PubMed
-
- Baek KJ, Thiel BA, Lucas S, Stuehr DJ. Macrophage nitric oxide synthase subunits. Purification, characterization, and role of prosthetic groups and substrate in regulating their association into a dimeric enzyme. J Biol Chem 268: 21120–21129, 1993 - PubMed
-
- Balasubramaniam V, Maxey AM, Morgan DB, Markham NE, Abman SH. Inhaled NO restores lung structure in eNOS-deficient mice recovering from neonatal hypoxia. Am J Physiol Lung Cell Mol Physiol 291: L119–L127, 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

