Dimensions of a projection column and architecture of VPM and POm axons in rat vibrissal cortex
- PMID: 20453248
- PMCID: PMC2936807
- DOI: 10.1093/cercor/bhq068
Dimensions of a projection column and architecture of VPM and POm axons in rat vibrissal cortex
Abstract
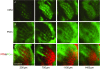
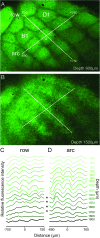
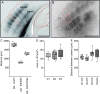
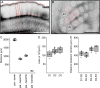
This is the first article in a series of 3 studies that investigate the anatomical determinants of thalamocortical (TC) input to excitatory neurons in a cortical column of rat primary somatosensory cortex (S1). S1 receives 2 major types of TC inputs, lemiscal and paralemniscal. Lemiscal axons arise from the ventral posteromedial nucleus (VPM) of the thalamus, whereas paralemniscal fibers originate in the posteromedial nucleus (POm). While these 2 TC projections are largely complementary in L4, overlap in other cortical layers is still a matter of debate. VPM and POm axons were specifically labeled in the same rat by virus-mediated expression of different fluorescent proteins. We show that columnar and septal projection patterns are maintained throughout most of the cortical depth with a lower degree of separation in infragranular layers, where TC axons form bands along rows. Finally, we present anatomical dimensions of "TC projection domains" for a standard column in S1.
Figures



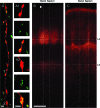
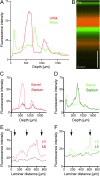
Comment in
-
Radial columns in cortical architecture: it is the composition that counts.Cereb Cortex. 2010 Oct;20(10):2261-4. doi: 10.1093/cercor/bhq127. Epub 2010 Jul 28. Cereb Cortex. 2010. PMID: 20667930 Free PMC article.
References
-
- Agmon A, Connors BW. Thalamocortical responses of mouse somatosensory (barrel) cortex in vitro. Neuroscience. 1991;41:365–379. - PubMed
-
- Ahissar E, Sosnik R, Bagdasarian K, Haidarliu S. Temporal frequency of whisker movement. II. Laminar organization of cortical representations. J Neurophysiol. 2001;86:354–367. - PubMed
-
- Alloway KD. Information processing streams in rodent barrel cortex: the differential functions of barrel and septal circuits. Cereb Cortex. 2008;18:979–989. - PubMed
-
- Alloway KD, Zhang M, Chakrabarti S. Septal columns in rodent barrel cortex: functional circuits for modulating whisking behavior. J Comp Neurol. 2004;480:299–309. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

