Deciphering the assembly of the Yersinia type III secretion injectisome
- PMID: 20453832
- PMCID: PMC2885934
- DOI: 10.1038/emboj.2010.84
Deciphering the assembly of the Yersinia type III secretion injectisome
Abstract
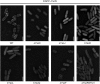
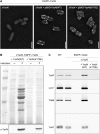
The assembly of the Yersinia enterocolitica type III secretion injectisome was investigated by grafting fluorescent proteins onto several components, YscC (outer-membrane (OM) ring), YscD (forms the inner-membrane (IM) ring together with YscJ), YscN (ATPase), and YscQ (putative C ring). The recombinant injectisomes were functional and appeared as fluorescent spots at the cell periphery. Epistasis experiments with the hybrid alleles in an array of injectisome mutants revealed a novel outside-in assembly order: whereas YscC formed spots in the absence of any other structural protein, formation of YscD foci required YscC, but not YscJ. We therefore propose that the assembly starts with YscC and proceeds through the connector YscD to YscJ, which was further corroborated by co-immunoprecipitation experiments. Completion of the membrane rings allowed the subsequent assembly of cytosolic components. YscN and YscQ attached synchronously, requiring each other, the interacting proteins YscK and YscL, but no further injectisome component for their assembly. These results show that assembly is initiated by the formation of the OM ring and progresses inwards to the IM ring and, finally, to a large cytosolic complex.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





References
-
- Abrahams JP, Leslie AG, Lutter R, Walker JE (1994) Structure at 2.8 A resolution of F1-ATPase from bovine heart mitochondria. Nature 370: 621–628 - PubMed
-
- Akeda Y, Galan JE (2005) Chaperone release and unfolding of substrates in type III secretion. Nature 437: 911–915 - PubMed
-
- Alfano JR, Collmer A (2004) Type III secretion system effector proteins: double agents in bacterial disease and plant defense. Annu Rev Phytopathol 42: 385–414 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

