Endoplasmic reticulum Ca2+ increases enhance mutant glucocerebrosidase proteostasis
- PMID: 20453863
- PMCID: PMC2873071
- DOI: 10.1038/nchembio.368
Endoplasmic reticulum Ca2+ increases enhance mutant glucocerebrosidase proteostasis
Erratum in
- Nat Chem Biol. 2010 Aug;6(8):630
Abstract
Altering intracellular calcium levels is known to partially restore mutant enzyme homeostasis in several lysosomal storage diseases, but why? We hypothesized that endoplasmic reticulum (ER) calcium increases enhance the folding, trafficking and function of these mutant misfolding- and degradation-prone lysosomal enzymes by increasing chaperone function. Here we report that increasing ER calcium levels by reducing ER calcium efflux through the ryanodine receptor, using antagonists or RNAi, or by promoting ER calcium influx by SERCA2b overexpression enhances mutant glucocerebrosidase (GC) homeostasis in cells derived from individuals with Gaucher's disease. Post-translational regulation of the calnexin folding pathway by an elevated ER calcium concentration seems to enhance the capacity of this chaperone system to fold mutant misfolding-prone enzymes, increasing the folded mutant GC population that can engage the trafficking receptor at the expense of ER-associated degradation, increasing the lysosomal GC concentration and activity.
Figures
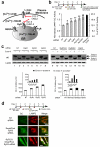
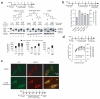


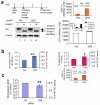

Comment in
-
Manipulating proteostasis.Nat Chem Biol. 2010 Jun;6(6):400-1. doi: 10.1038/nchembio.374. Nat Chem Biol. 2010. PMID: 20479748 No abstract available.
References
-
- Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. - PubMed
-
- Deuerling E, Bukau B. Chaperone-assisted folding of newly synthesized proteins in the cytosol. Crit. Rev. Biochem. Mol. Biol. 2004;39:261–277. - PubMed
-
- Bukau B, Weissman J, Horwich A. Molecular chaperones and protein quality control. Cell. 2006;125:443–451. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

