WNT4/beta-catenin pathway maintains female germ cell survival by inhibiting activin betaB in the mouse fetal ovary
- PMID: 20454446
- PMCID: PMC2861588
- DOI: 10.1371/journal.pone.0010382
WNT4/beta-catenin pathway maintains female germ cell survival by inhibiting activin betaB in the mouse fetal ovary
Abstract
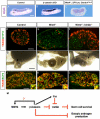
Female germ cells are essential for organogenesis of the ovary; without them, ovarian follicles do not form and functional and structural characteristics of the ovary are lost. We and others showed previously that when either Wnt4 or beta-catenin was inactivated in the fetal ovary, female germ cells underwent degeneration. In this study, we set out to understand whether these two factors belong to the same pathway and how they maintain female germ cell survival. We found that activation of beta-catenin in somatic cells in the Wnt4 knockout ovary restored germ cell numbers, placing beta-catenin downstream of WNT4. In the absence of Wnt4 or beta-catenin, female germ cells entered meiosis properly; however, they underwent apoptosis afterwards. Activin betaB (Inhbb), a subunit of activins, was upregulated in the Wnt4 and beta-catenin knockout ovaries, suggesting that Inhbb could be the cause for the loss of female germ cells, which are positive for activin receptors. Indeed, removal of Inhbb in the Wnt4 knockout ovaries prevented female germ cells from undergoing degeneration. We conclude that WNT4 maintains female germ cell survival by inhibiting Inhbb expression via beta-catenin in the somatic cells. Maintenance of female germ cells hinge upon a delicate balance between positive (WNT4 and beta-catenin) and negative (activin betaB) regulators derived from the somatic cells in the fetal ovary.
Conflict of interest statement
Figures

References
-
- Ruggiu M, Speed R, Taggart M, McKay SJ, Kilanowski F, et al. The mouse Dazla gene encodes a cytoplasmic protein essential for gametogenesis. Nature. 1997;389:73–77. - PubMed
-
- McNeilly JR, Saunders PT, Taggart M, Cranfield M, Cooke HJ, et al. Loss of oocytes in Dazl knockout mice results in maintained ovarian steroidogenic function but altered gonadotropin secretion in adult animals. Endocrinology. 2000;141:4284–4294. - PubMed
-
- Soyal SM, Amleh A, Dean J. FIGalpha, a germ cell-specific transcription factor required for ovarian follicle formation. Development. 2000;127:4645–4654. - PubMed
-
- Hashimoto N, Kubokawa R, Yamazaki K, Noguchi M, Kato Y. Germ cell deficiency causes testis cord differentiation in reconstituted mouse fetal ovaries. J Exp Zool. 1990;253:61–70. - PubMed
-
- Behringer RR, Cate RL, Froelick GJ, Palmiter RD, Brinster RL. Abnormal sexual development in transgenic mice chronically expressing mullerian inhibiting substance. Nature. 1990;345:167–170. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

