14-3-3Tau regulates Beclin 1 and is required for autophagy
- PMID: 20454448
- PMCID: PMC2861590
- DOI: 10.1371/journal.pone.0010409
14-3-3Tau regulates Beclin 1 and is required for autophagy
Abstract
Background: Beclin 1 plays an essential role in autophagy; however, the regulation of Beclin 1 expression remains largely unexplored. An earlier ChIP-on-chip study suggested Beclin 1 could be an E2F target. Previously, we also reported that 14-3-3tau regulates E2F1 stability, and is required for the expression of several E2F1 target genes. 14-3-3 proteins mediate many cellular signaling processes, but its role in autophagy has not been investigated. We hypothesize that 14-3-3tau could regulate Beclin 1 expression through E2F1 and thus regulate autophagy.
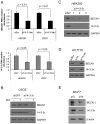
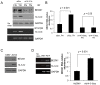
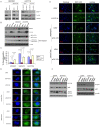
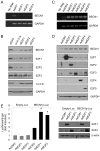
Methods and findings: Using the RNAi technique we demonstrate a novel role for one of 14-3-3 isoforms, 14-3-3tau, in the regulation of Beclin 1 expression and autophagy. Depletion of 14-3-3tau inhibits the expression of Beclin 1 in many different cell lines; whereas, upregulation of 14-3-3tau induces Beclin 1. The regulation is physiologically relevant as an extracellular matrix protein tenascin-C, a known 14-3-3tau inducer, can induce Beclin 1 through 14-3-3tau. Moreover, rapamycin-induced, serum free-induced and amino acid starvation-induced autophagy depends on 14-3-3tau. We also show the expression of Beclin 1 depends on E2F, and E2F can transactivate the Beclin 1 promoter in a promoter reporter assay. Upregulation of Beclin 1 by 14-3-3tau requires E2F1. Depletion of E2F1, like 14-3-3tau, also inhibits autophagy.
Conclusion: Taken together, this study uncovers a role for 14-3-3tau in Beclin 1 and autophagy regulation probably through regulation of E2F1.
Conflict of interest statement
Figures
Similar articles
-
Hepatitis B virus X protein sensitizes cells to starvation-induced autophagy via up-regulation of beclin 1 expression.Hepatology. 2009 Jan;49(1):60-71. doi: 10.1002/hep.22581. Hepatology. 2009. PMID: 19065679
-
E2F1 regulates autophagy and the transcription of autophagy genes.Oncogene. 2008 Aug 14;27(35):4860-4. doi: 10.1038/onc.2008.117. Epub 2008 Apr 14. Oncogene. 2008. PMID: 18408756
-
E2F-1 induces melanoma cell apoptosis via PUMA up-regulation and Bax translocation.BMC Cancer. 2007 Jan 30;7:24. doi: 10.1186/1471-2407-7-24. BMC Cancer. 2007. PMID: 17263886 Free PMC article.
-
Impaired autophagy and APP processing in Alzheimer's disease: The potential role of Beclin 1 interactome.Prog Neurobiol. 2013 Jul-Aug;106-107:33-54. doi: 10.1016/j.pneurobio.2013.06.002. Epub 2013 Jul 1. Prog Neurobiol. 2013. PMID: 23827971 Review.
-
Beclin 1 biology and its role in heart disease.Curr Cardiol Rev. 2015;11(3):229-37. doi: 10.2174/1573403x10666141106104606. Curr Cardiol Rev. 2015. PMID: 25373623 Free PMC article. Review.
Cited by
-
14-3-3 proteins-a moonlight protein complex with therapeutic potential in neurological disorder: in-depth review with Alzheimer's disease.Front Mol Biosci. 2024 Feb 5;11:1286536. doi: 10.3389/fmolb.2024.1286536. eCollection 2024. Front Mol Biosci. 2024. PMID: 38375509 Free PMC article. Review.
-
Regulation of autophagic activity by 14-3-3ζ proteins associated with class III phosphatidylinositol-3-kinase.Cell Death Differ. 2011 Mar;18(3):479-92. doi: 10.1038/cdd.2010.118. Epub 2010 Oct 1. Cell Death Differ. 2011. PMID: 20885446 Free PMC article.
-
XBP1 mRNA splicing triggers an autophagic response in endothelial cells through BECLIN-1 transcriptional activation.J Biol Chem. 2013 Jan 11;288(2):859-72. doi: 10.1074/jbc.M112.412783. Epub 2012 Nov 26. J Biol Chem. 2013. PMID: 23184933 Free PMC article.
-
Neural stem cell small extracellular vesicle-based delivery of 14-3-3t reduces apoptosis and neuroinflammation following traumatic spinal cord injury by enhancing autophagy by targeting Beclin-1.Aging (Albany NY). 2019 Sep 28;11(18):7723-7745. doi: 10.18632/aging.102283. Epub 2019 Sep 28. Aging (Albany NY). 2019. PMID: 31563124 Free PMC article.
-
Mesenchymal Stem Cell/Multipotent Stromal Cell Augmentation of Wound Healing: Lessons from the Physiology of Matrix and Hypoxia Support.Am J Pathol. 2020 Jul;190(7):1370-1381. doi: 10.1016/j.ajpath.2020.03.017. Epub 2020 Apr 12. Am J Pathol. 2020. PMID: 32294456 Free PMC article. Review.
References
-
- Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. - PubMed
-
- Pacheco CD, Kunkel R, Lieberman AP. Autophagy in Niemann-Pick C disease is dependent upon Beclin-1 and responsive to lipid trafficking defects. Hum Mol Genet. 2007;16:1495–1503. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

