Effectiveness of multidrug antiretroviral regimens to prevent mother-to-child transmission of HIV-1 in routine public health services in Cameroon
- PMID: 20454459
- PMCID: PMC2861601
- DOI: 10.1371/journal.pone.0010411
Effectiveness of multidrug antiretroviral regimens to prevent mother-to-child transmission of HIV-1 in routine public health services in Cameroon
Abstract
Background: Multidrug antiretroviral (ARV) regimens including HAART and short-course dual antiretroviral (sc-dARV) regimens were introduced in 2004 to improve Prevention of Mother-to-Child Transmission (PMTCT) in Cameroon. We assessed the effectiveness of these regimens from 6-10 weeks and 12 months of age, respectively.
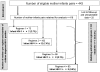
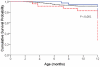
Methodology/findings: We conducted a retrospective cohort study covering the period from October 2004 to March 2008 in a reference hospital in Cameroon. HIV-positive pregnant women with CD4 < or = 350 cells/mm(3) received first-line HAART [regimen 1] while the others received ARV prophylaxis including sc-dARV or single dose nevirapine (sd-NVP). Sc-dARV included at least two drugs according to different gestational ages: zidovudine (ZDV) from 28-32 weeks plus sd-NVP [regimen 2], ZDV and lamuvidine (3TC) from 33-36 weeks plus sd-NVP [regimen 3]. When gestational age was > or = 37 weeks, women received sd-NVP during labour [regimen 4]. Infants received sd-NVP plus ZDV and 3TC for 7 days or 30 days. Early diagnosis (6-10 weeks) was done, using b-DNA and subsequently RT-PCR. We determined early MTCT rate and associated risk factors using logistic regression. The 12-month HIV-free survival was assessed using Cox regression. Among 418 mothers, 335 (80%) received multidrug ARV regimens (1, 2, and 3) and MTCT rate with multidrug regimens was 6.6% [95%CI: 4.3-9.6] at 6 weeks, without any significant difference between regimens. Duration of mother's ARV regimen < 4 weeks [OR = 4.7, 95%CI: 1.3-17.6], mother's CD4 < 350 cells/mm(3) [OR = 6.4, 95%CI: 1.8-22.5] and low birth weight [OR = 4.0, 95%CI: 1.4-11.3] were associated with early MTCT. By 12 months, mixed feeding [HR = 8.7, 95%CI: 3.6-20.6], prematurity [HR = 2.3, 95%CI: 1.2-4.3] and low birth weight were associated with children's risk of progressing to infection or death.
Conclusions: Multidrug ARV regimens for PMTCT are feasible and effective in routine reference hospital. Early initiation of ARV during pregnancy and proper obstetrical care are essential to improve PMTCT.
Conflict of interest statement
Figures
References
-
- Tindyebwa D, Kayita J, Musoke P, Eley B, Nduati R. Handbook on Paediatric AIDS in Africa. Kampala: ANECCA; 2006. 260
-
- UNAIDS. AIDS epidemic update: December 2005. Geneva: UNAIDS; 2005.
-
- UNAIDS. AIDS epidemic update: December 2007. Geneva: UNAIDS; 2007.
-
- Mofenson LM, McIntyre JA. Advances and research directions in the prevention of mother-to-child HIV-1 transmission. Lancet. 2000;355:2237–2244. - PubMed
-
- Coutsoudis A, Goga AE, Rollins N, Coovadia HM on behalf of the child health Group. Free formula milk for infants of HIV-infected women: blessing or curse? Health Policy Plan. 2002;17:154–160. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

