Factor VII deficiency impairs cutaneous wound healing in mice
- PMID: 20454518
- PMCID: PMC2864811
- DOI: 10.2119/molmed.2009.00171
Factor VII deficiency impairs cutaneous wound healing in mice
Abstract
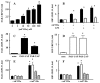
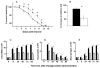
Skin keratinocytes express tissue factor (TF) and are highly associated with skin wound healing. Although it has been demonstrated that perivascular TF expression in granulation tissue formed after dermal injury is downregulated during healing, studies of the mechanism of factor (F) VII, a TF ligand, in skin wound healing are lacking. We reported the use of a dermal punch model to demonstrate that low-expressing FVII mice (approximately 1% of wild type [WT]) exhibited impaired skin wound healing compared with WT controls. These low-FVII mice showed defective reepithelialization and reduced inflammatory cell infiltration at wound sites. This attenuated reepithelialization was associated with diminished expression of the transcription factor early growth response 1 (Egr-1). In vitro, Egr-1 was shown to be essential for the FVIIa-induced regulation of keratinocyte migration and inflammation. Both Egr-1 upregulation and downstream inflammatory cytokine appearance in keratinocytes depended on FVIIa/TF/protease-activated receptor 2 (PAR-2)-induced signaling and did not require subsequent generation of FXa and thrombin. The participation of Egr-1 in FVIIa-mediated regulation of keratinocyte function was confirmed by use of Egr-1-deficient mice, wherein a significant delay in skin wound healing after injury was observed, relative to WT mice. The results from these studies demonstrate an in vivo mechanistic relationship between FVIIa, Egr-1 and the inflammatory response in keratinocyte function during the wound healing process.
Figures


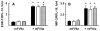
Similar articles
-
The endothelial protein C receptor supports tissue factor ternary coagulation initiation complex signaling through protease-activated receptors.J Biol Chem. 2011 Feb 18;286(7):5756-67. doi: 10.1074/jbc.M110.201228. Epub 2010 Dec 13. J Biol Chem. 2011. PMID: 21149441 Free PMC article.
-
Spontaneous skin erosions and reduced skin and corneal wound healing characterize CLIC4(NULL) mice.Am J Pathol. 2012 Jul;181(1):74-84. doi: 10.1016/j.ajpath.2012.03.025. Epub 2012 May 18. Am J Pathol. 2012. PMID: 22613027 Free PMC article.
-
A coagulation factor VII deficiency protects against acute inflammatory responses in mice.J Pathol. 2006 Dec;210(4):488-96. doi: 10.1002/path.2073. J Pathol. 2006. PMID: 17054310
-
Active site-inhibited seven: mechanism of action including signal transduction.Semin Hematol. 2001 Oct;38(4 Suppl 12):39-42. doi: 10.1016/s0037-1963(01)90146-5. Semin Hematol. 2001. PMID: 11735109 Review.
-
Expression and function of keratinocyte growth factor and activin in skin morphogenesis and cutaneous wound repair.J Investig Dermatol Symp Proc. 2000 Dec;5(1):34-9. doi: 10.1046/j.1087-0024.2000.00009.x. J Investig Dermatol Symp Proc. 2000. PMID: 11147673 Review.
Cited by
-
Innate immunity, hemostasis and matrix remodeling: PTX3 as a link.Semin Immunol. 2016 Dec;28(6):570-577. doi: 10.1016/j.smim.2016.10.012. Epub 2016 Nov 20. Semin Immunol. 2016. PMID: 27881292 Free PMC article. Review.
-
Bafilomycin A1 Accelerates Chronic Refractory Wound Healing in db/db Mice.Biomed Res Int. 2020 Jul 2;2020:6265701. doi: 10.1155/2020/6265701. eCollection 2020. Biomed Res Int. 2020. PMID: 32714982 Free PMC article.
-
Upregulation of the coagulation factor VII gene during glucose deprivation is mediated by activating transcription factor 4.PLoS One. 2012;7(7):e40994. doi: 10.1371/journal.pone.0040994. Epub 2012 Jul 27. PLoS One. 2012. PMID: 22848420 Free PMC article.
-
Protease-activated receptor 2 signaling in inflammation.Semin Immunopathol. 2012 Jan;34(1):133-49. doi: 10.1007/s00281-011-0289-1. Epub 2011 Oct 6. Semin Immunopathol. 2012. PMID: 21971685 Review.
-
Gallium-modified gelatin nanoparticles loaded with quercetin promote skin wound healing via the regulation of bacterial proliferation and macrophage polarization.Front Bioeng Biotechnol. 2023 Jan 26;11:1124944. doi: 10.3389/fbioe.2023.1124944. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 36777248 Free PMC article.
References
-
- Holmdahl L, Ivarsson ML. The role of cytokines, coagulation, and fibrinolysis in peritoneal tissue repair. Eur J Surg. 1999;165:1012–9. - PubMed
-
- Stadelmann WK, Digenis AG, Tobin GR. Physiology and healing dynamics of chronic cutaneous wounds. Am J Surg. 1998;176:26S–38S. - PubMed
-
- Dovi JV, Szpaderska AM, DiPietro LA. Neutrophil function in the healing wound: adding insult to injury. Thromb Haemost. 2004;92:275–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

