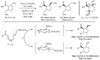
Direct Copper-Free Domino Conjugate Addition-Cycloallylation using Organozinc Reagents
- PMID: 20454546
- PMCID: PMC2865154
- DOI: 10.1002/adsc.200800242
Direct Copper-Free Domino Conjugate Addition-Cycloallylation using Organozinc Reagents
Abstract
The Direct Approach: Enones possessing appendant allylic carbonates react directly with diorganozinc reagents in the presence of zinc diiodide [ZnI(2)] to provide 5- and 6-membered ring products of tandem or domino conjugate addition-cycloallylation in good to excellent yield. In a related copper-free transformation, allylic carbonates are found to engage in direct allylic substitution with diorganozinc reagents.
Figures
Similar articles
-
Radical-polar crossover domino reactions involving organozinc and mixed organocopper/organozinc reagents.Chemistry. 2006 Aug 25;12(25):6506-13. doi: 10.1002/chem.200600334. Chemistry. 2006. PMID: 16789032
-
Conjugate addition of mixed diorganozinc compounds and functionalized organozinc cuprates to nitroolefins.Org Lett. 2002 Sep 19;4(19):3289-91. doi: 10.1021/ol026557w. Org Lett. 2002. PMID: 12227771
-
Efficient Synthesis of All-Carbon Quaternary Centers via the Conjugate Addition of Functionalized Monoorganozinc Bromides.J Vis Exp. 2019 May 26;(147). doi: 10.3791/59775. J Vis Exp. 2019. PMID: 31180356
-
Highly anti-selective SN2' substitutions of chiral cyclic 2-iodo-allylic alcohol derivatives with mixed zinc-copper reagents.Org Lett. 2003 Apr 3;5(7):1059-61. doi: 10.1021/ol0340742. Org Lett. 2003. PMID: 12659573
-
Phosphoramidites: marvellous ligands in catalytic asymmetric conjugate addition.Acc Chem Res. 2000 Jun;33(6):346-53. doi: 10.1021/ar990084k. Acc Chem Res. 2000. PMID: 10891052 Review.
Cited by
-
Stereogenic-at-metal Zn- and Al-based N-heterocyclic carbene (NHC) complexes as bifunctional catalysts in Cu-free enantioselective allylic alkylations.J Am Chem Soc. 2009 Aug 19;131(32):11625-33. doi: 10.1021/ja904654j. J Am Chem Soc. 2009. PMID: 19630406 Free PMC article.
References
-
-
For selected reviews on “tandem,” “cascade” or “domino” transformations, see: Tietze LF, Beifuss U. Angew. Chem. 1993;105:137–170. Angew. Chem. Int. Ed. Engl. 1993;32:131–163. Tietze LF. Chem. Rev. 1996;96:115–136. Mayer SF, Kroutil W, Faber K. Chem. Soc. Rev. 2001;30:332. Nicolaou KC, Montagnon T, Snyder SA. Chem. Commun. 2003:551–564. Pellissier H. Tetrahedron. 2006;62:2143–2173. Chapman CJ, Frost CG. Synthesis. 2007:1–21. Walji AM, MacMillan DWC. Synlett. 2007:1477–1489. Miura T, Murakami M. Chem. Commun. 2007:217–224.
-
-
-
For selected reviews on stoichiometric conjugate addition-electrophilic trapping, see: Suzuki M, Noyori R. Organocopper Reagents. 1994:185–216. Taylor RJK. Synthesis. 1985:364–392. Chapdelaine MJ, Hulce M. Org. React. 1990;38:225–653. Ihara M, Fukumoto K. Angew. Chem. 1993;105:1059–1071. Angew. Chem. Int. Ed. Engl. 1993;32:1010–1022.
-
-
-
For selected reviews on metal catalyzed conjugate addition-electrophilic trapping, see: Balme G, Bouyssi D, Monteiro N. Top. Organomet. Chem. 2006;19:115–148. Guo H-C, Ma J-A. Angew. Chem. 2006;118:362–375. Angew. Chem. Int. Ed. 2006;45:354–366. Christoffers J, Koripelly G, Rosiak A, Rössle M. Synthesis. 2007:1279–1300.
-
-
-
For Co-catalyzed 1,4-reduction-aldol and Michael cyclization, see: Baik T-G, Luiz A-L, Wang L-C, Krische MJ. J. Am. Chem. Soc. 2001;123:5112–5113. Wang L-C, Jang H-Y, Roh Y, Schultz AJ, Wang X, Lynch V, Krische MJ. J. Am. Chem. Soc. 2002;124:9448–9453.
-
-
-
For Rh-catalyzed 1,4-reduction-aldol cyclization, see: Jang H-Y, Huddleston RR, Krische MJ. J. Am. Chem. Soc. 2002;124:15156–15157. Huddleston RR, Krische MJ. Org. Lett. 2003;5:1143–1146. Huddleston RR, Cauble DF, Krische MJ. J. Org. Chem. 2003;68:11–14. Koech P, Krische MJ. Org. Lett. 2004;6:691–694.
-
Grants and funding
LinkOut - more resources
Full Text Sources