Secreted Mycobacterium tuberculosis Rv3654c and Rv3655c proteins participate in the suppression of macrophage apoptosis
- PMID: 20454556
- PMCID: PMC2864267
- DOI: 10.1371/journal.pone.0010474
Secreted Mycobacterium tuberculosis Rv3654c and Rv3655c proteins participate in the suppression of macrophage apoptosis
Abstract
Background: Inhibition of macrophage apoptosis by Mycobacterium tuberculosis has been proposed as one of the virulence mechanisms whereby the pathogen avoids the host defense. The mechanisms by which M. tuberculosis H37Rv strain suppress apoptosis and escapes human macrophage killing was investigated.
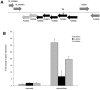
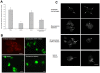
Methodology/principal findings: The screening of a transposon mutant bank identified several mutants, which, in contrast to the wild-type bacterium, had impaired ability to inhibit apoptosis of macrophages. Among the identified genes, Rv3659c (31G12 mutant) belongs to an operon reminiscent of type IV pili. The Rv3654c and Rv3655c putative proteins in a seven-gene operon are secreted into the macrophage cytoplasm and suppress apoptosis by blocking the extrinsic pathway. The operon is highly expressed when the bacterium is within macrophages, compared to the expression level in the extracellular environment. Rv3654c recognizes the polypyrimidine tract binding Protein-associated Splicing Factor (PSF) and cleaves it, diminishing the availability of caspase-8. While M. tuberculosis inhibits apoptosis by the extrinsic pathway, the pathogen does not appear to affect the intrinsic pathway. Inactivation of the intrinsic pathway by pharmacologic agents afftects M. tuberculosis and induces cell necrosis. Likewise, inactivation of PSF by siRNA significantly decreased the level of caspase-8 in macrophages.
Conclusion: While M. tuberculosis inhibits the extrinsic pathway of apoptosis, it appears to activate the intrinsic pathway leading to macrophage necrosis as a potential exit strategy.
Conflict of interest statement
Figures




References
-
- Ting LM, Kim AC, Cattamanchi A, Ernst JD. Mycobacterium tuberculosis inhibits IFN-gamma transcriptional responses without inhibiting activation of STAT1. J Immunol. 1999;163:3898–3906. - PubMed
-
- Sturgill-Koszycki S, Schlesinger PH, Chakraborty P, Haddix PL, Collins HL, et al. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science. 1994;263:678–681. - PubMed
-
- Via LE, Deretic D, Ulmer RJ, Hibler NS, Huber LA, et al. Arrest of mycobacterial phagosome maturation is caused by a block in vesicle fusion between stages controlled by rab5 and rab7. J Biol Chem. 1997;272:13326–13331. - PubMed
-
- Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

