Sex and the single cell. II. There is a time and place for sex
- PMID: 20454565
- PMCID: PMC2864297
- DOI: 10.1371/journal.pbio.1000365
Sex and the single cell. II. There is a time and place for sex
Abstract
The Drosophila melanogaster sex hierarchy controls sexual differentiation of somatic cells via the activities of the terminal genes in the hierarchy, doublesex (dsx) and fruitless (fru). We have targeted an insertion of GAL4 into the dsx gene, allowing us to visualize dsx-expressing cells in both sexes. Developmentally and as adults, we find that both XX and XY individuals are fine mosaics of cells and tissues that express dsx and/or fruitless (fru(M)), and hence have the potential to sexually differentiate, and those that don't. Evolutionary considerations suggest such a mosaic expression of sexuality is likely to be a property of other animal species having two sexes. These results have also led to a major revision of our view of how sex-specific functions are regulated by the sex hierarchy in flies. Rather than there being a single regulatory event that governs the activities of all downstream sex determination regulatory genes-turning on Sex lethal (Sxl) RNA splicing activity in females while leaving it turned off in males-there are, in addition, elaborate temporal and spatial transcriptional controls on the expression of the terminal regulatory genes, dsx and fru. Thus tissue-specific aspects of sexual development are jointly specified by post-transcriptional control by Sxl and by the transcriptional controls of dsx and fru expression.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
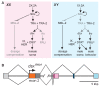
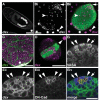

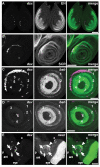
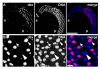

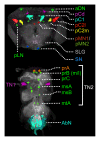
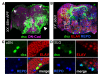
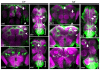
Comment in
-
Sex on the fly.PLoS Biol. 2010 May 4;8(5):e1000364. doi: 10.1371/journal.pbio.1000364. PLoS Biol. 2010. PMID: 20454562 Free PMC article. No abstract available.
References
-
- Cline T. W, Meyer B. J. Vive la difference: males vs females in flies vs worms. Annu Rev Genet. 1996;30:637–702. - PubMed
-
- Christiansen A. E, Keisman E. L, Ahmad S. M, Baker B. S. Sex comes in from the cold: the integration of sex and pattern. Trends Genet. 2002;18:510–516. - PubMed
-
- Marin I, Baker B. S. The evolutionary dynamics of sex determination. Science. 1998;281:1990–1994. - PubMed
-
- Manoli D. S, Meissner G. W, Baker B. S. Blueprints for behavior: genetic specification of neural circuitry for innate behaviors. Trends Neurosci. 2006;29:444–451. - PubMed
-
- Billeter J. C, Rideout E. J, Dornan A. J, Goodwin S. F. Control of male sexual behavior in Drosophila by the sex determination pathway. Curr Biol. 2006;16:R766–R776. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

