ISOLATION OF CAFFEIC ACID FROM EUPATORIUM ADENOPHORUM SPRENG BY HIGH-SPEED COUNTERCURRENT CHROMATOGRAPHY AND SYNTHESIS OF CAFFEIC ACID-INTERCALATED LAYERED DOUBLE HYDROXIDE
- PMID: 20454592
- PMCID: PMC2863339
- DOI: 10.1080/10826071003684471
ISOLATION OF CAFFEIC ACID FROM EUPATORIUM ADENOPHORUM SPRENG BY HIGH-SPEED COUNTERCURRENT CHROMATOGRAPHY AND SYNTHESIS OF CAFFEIC ACID-INTERCALATED LAYERED DOUBLE HYDROXIDE
Abstract
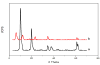
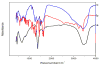
Preparative high-speed countercurrent chromatography (HSCCC) was successfully used for isolation and purification of caffeic acid from Eupatorium adenophorum Spreng with a solvent system composed of ethyl acetate-methanol-water at a volume ratio of 10:1:10, v/v. Using a preparative unit of the HSCCC centrifuge, about a 938 mg amount of the crude extract was separated, yielding 63.2 mg of caffeic acid at purity of 96.0%. Then, the anti-microbial and anti-virus drug caffeic acid (C(9)H(8)O(4)) was intercalated into layered double hydroxides for the first time by anion exchange under a nitrogen atmosphere. The product caffeic acid-LDH has been characterized by powder X-ray diffraction (XRD), Fourier transform-infrared (FT-IR), Scanning electron micrographs (SEM), indicating that the drug has been successfully intercalated into LDH.
Figures



References
-
- Zhao XB, Zhang LH, Liu DH. Bioresource Technol. 2008;99:3729–3736. - PubMed
-
- Gu WB, Sang WG, Ling HB, Axmacher JC. Agriculture, Ecosystems and Environment. 2008;124:173–178.
-
- Wang L, Qin RHJ. Southwest forestry college. 2004;24:72–75.
-
- Horn AF, Nielsen NS, Jacobsen C. Food Chem. 2009;112:412–420.
-
- Gutzeit D, Winterhalter P, Jerz GJ. Chromatogr A. 2007;1172:40–46. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
