In mice, tuberculosis progression is associated with intensive inflammatory response and the accumulation of Gr-1 cells in the lungs
- PMID: 20454613
- PMCID: PMC2864263
- DOI: 10.1371/journal.pone.0010469
In mice, tuberculosis progression is associated with intensive inflammatory response and the accumulation of Gr-1 cells in the lungs
Abstract
Background: Infection with Mycobacterium tuberculosis (Mtb) results in different clinical outcomes ranging from asymptomatic containment to rapidly progressing tuberculosis (TB). The mechanisms controlling TB progression in immunologically-competent hosts remain unclear.
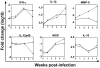
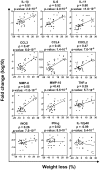
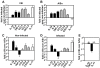
Methodology/principal findings: To address these mechanisms, we analyzed TB progression in a panel of genetically heterogeneous (A/SnxI/St) F2 mice, originating from TB-highly-susceptible I/St and more resistant A/Sn mice. In F2 mice the rates of TB progression differed. In mice that did not reach terminal stage of infection, TB progression did not correlate with lung Mtb loads. Nor was TB progression correlated with lung expression of factors involved in antibacterial immunity, such as iNOS, IFN-gamma, or IL-12p40. The major characteristics of progressing TB was high lung expression of the inflammation-related factors IL-1beta, IL-6, IL-11 (p<0.0003); CCL3, CCL4, CXCL2 (p<0.002); MMP-8 (p<0.0001). The major predictors of TB progression were high expressions of IL-1beta and IL-11. TNF-alpha had both protective and harmful effects. Factors associated with TB progression were expressed mainly by macrophages (F4-80(+) cells) and granulocytes (Gr-1(hi)/Ly-6G(hi) cells). Macrophages and granulocytes from I/St and A/Sn parental strains exhibited intrinsic differences in the expression of inflammatory factors, suggesting that genetically determined peculiarities of phagocytes transcriptional response could account for the peculiarities of gene expression in the infected lungs. Another characteristic feature of progressing TB was the accumulation in the infected lungs of Gr-1(dim) cells that could contribute to TB progression.
Conclusions/significance: In a population of immunocompetent hosts, the outcome of TB depends on quantitatively- and genetically-controlled differences in the intensity of inflammatory responses, rather than being a direct consequence of mycobacterial colonization. Local accumulation of Gr-1(dim) cells is a newly identified feature of progressing TB. High expression of IL-1beta and IL-11 are potential risk factors for TB progression and possible targets for TB immunomodulation.
Conflict of interest statement
Figures




References
-
- Raviglione MC. The TB epidemic from 1992 to 2002. Tuberculosis (Edinb) 2003;83:4–14. - PubMed
-
- Bellamy R, Hill AVS. Host genetic susceptibility to human tuberculosis. Novartis Foundation Symposium. 1998;217:3–23. - PubMed
-
- Dietrich WF. Using mouse genetics to understand infectious disease pathogenesis. Genome Res. 2001;11:325–331. - PubMed
-
- Flynn JL, Ernst JD. Immune responses in tuberculosis. Curr Opin Immunol. 2000;12:432–436. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

