Transcriptional Regulation of the rsbV Promoter Controlling Stress Responses to Ethanol, Carbon Limitation, and Phosphorous Limitation in Bacillus subtilis
- PMID: 20454630
- PMCID: PMC2862951
- DOI: 10.1155/2010/263410
Transcriptional Regulation of the rsbV Promoter Controlling Stress Responses to Ethanol, Carbon Limitation, and Phosphorous Limitation in Bacillus subtilis
Abstract
The sigma(B)-dependent promoter in front of the rsbV gene of Bacillus subtilis is induced approximately 5-fold in response to (1) the addition of 4% ethanol, (2) carbon starvation, and (3) phosphorous starvation. Binding sites for the global carbon and nitrogen regulators, CcpA and TnrA, were mutated, and the consequences of their loss and that of CcpA or TnrA were studied using rsbV-lacZ fusions. These responses proved to be dependent on CcpA, TnrA, and their putative binding sites upstream of the promoter. Induction in response to glucose limitation was largely abolished by loss of CcpA or the upstream region, while induction in response to phosphorous limitation was largely abolished only by the upstream mutations. The results suggest that CcpA directly influences the carbon starvation response and that both proteins exert indirect effects on all three stress responses. The integrity of the DNA sequence is important for all three responses.
Figures

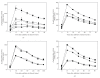
 ); hprK mutant (×); the rsbV1m1 mutant (◆); rsbV3 (lacking the TnrA binding site) (▾) rsbV5 (lacking the TnrA and CcpA binding sites) (▴). (b) Effects of the loss of CcpA (
); hprK mutant (×); the rsbV1m1 mutant (◆); rsbV3 (lacking the TnrA binding site) (▾) rsbV5 (lacking the TnrA and CcpA binding sites) (▴). (b) Effects of the loss of CcpA ( ), TnrA (▴), or both transcription factors (◆) on the ethanol stress response of the σ
B-dependent promoter in the rsb operon. (c) Effect of glucose on the ethanol stress response of the rsbV promoter. The experiment was conducted in LB medium with (closed symbols) and without (open symbols) 1% glucose. The wild type (squares) and ccpA mutant (circles) were examined. (d) Response of the σ
B-dependent ctc gene to ethanol stress. A lacZ fusion was made to the ctc gene, and the response to 4% ethanol was measured in the wild-type background (■), the ccpA mutant background (
), TnrA (▴), or both transcription factors (◆) on the ethanol stress response of the σ
B-dependent promoter in the rsb operon. (c) Effect of glucose on the ethanol stress response of the rsbV promoter. The experiment was conducted in LB medium with (closed symbols) and without (open symbols) 1% glucose. The wild type (squares) and ccpA mutant (circles) were examined. (d) Response of the σ
B-dependent ctc gene to ethanol stress. A lacZ fusion was made to the ctc gene, and the response to 4% ethanol was measured in the wild-type background (■), the ccpA mutant background ( ), the rsbV1m1 genetic background (◆), and the rsbV5 genetic background (▴). The experiment was conducted in LB medium under standard conditions. Ethanol (4%) was added at t = 0.
), the rsbV1m1 genetic background (◆), and the rsbV5 genetic background (▴). The experiment was conducted in LB medium under standard conditions. Ethanol (4%) was added at t = 0.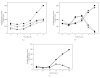
 ), tnrA (◆), and ccpA tnrA (▴) double knockout mutants on the glucose starvation response as a function of time. The wild-type response is represented by square symbols (■). (c) Effect of glucose limitation on expression of the ctc gene which is under sigma B (σ
B) control, in the wild-type (■) or ccpA (
), tnrA (◆), and ccpA tnrA (▴) double knockout mutants on the glucose starvation response as a function of time. The wild-type response is represented by square symbols (■). (c) Effect of glucose limitation on expression of the ctc gene which is under sigma B (σ
B) control, in the wild-type (■) or ccpA ( ) genetic backgrounds.
) genetic backgrounds.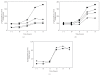
 ), TnrA (◆), and both transcription factors (▴) as compared with wild type (■) on the phosphate limitation response. (c) Lack of effect of the loss of CcpA (
), TnrA (◆), and both transcription factors (▴) as compared with wild type (■) on the phosphate limitation response. (c) Lack of effect of the loss of CcpA ( ) on the phosphate limitation response of the ctc gene which is under sigma B (σ
B) control. The wild-type response is represented by ■.
) on the phosphate limitation response of the ctc gene which is under sigma B (σ
B) control. The wild-type response is represented by ■.Similar articles
-
Elaborate transcription regulation of the Bacillus subtilis ilv-leu operon involved in the biosynthesis of branched-chain amino acids through global regulators of CcpA, CodY and TnrA.Mol Microbiol. 2005 Jun;56(6):1560-73. doi: 10.1111/j.1365-2958.2005.04635.x. Mol Microbiol. 2005. PMID: 15916606
-
Reactivation of the Bacillus subtilis anti-sigma B antagonist, RsbV, by stress- or starvation-induced phosphatase activities.J Bacteriol. 1996 Sep;178(18):5456-63. doi: 10.1128/jb.178.18.5456-5463.1996. J Bacteriol. 1996. PMID: 8808936 Free PMC article.
-
Mutational analysis of the TnrA-binding sites in the Bacillus subtilis nrgAB and gabP promoter regions.J Bacteriol. 1998 Jun;180(11):2943-9. doi: 10.1128/JB.180.11.2943-2949.1998. J Bacteriol. 1998. PMID: 9603886 Free PMC article.
-
The role of CcpA transcriptional regulator in carbon metabolism in Bacillus subtilis.FEMS Microbiol Lett. 1996 Jan 1;135(1):9-15. doi: 10.1111/j.1574-6968.1996.tb07959.x. FEMS Microbiol Lett. 1996. PMID: 8598282 Review.
-
Control of key metabolic intersections in Bacillus subtilis.Nat Rev Microbiol. 2007 Dec;5(12):917-27. doi: 10.1038/nrmicro1772. Nat Rev Microbiol. 2007. PMID: 17982469 Review.
Cited by
-
6S-2 RNA deletion in the undomesticated B. subtilis strain NCIB 3610 causes a biofilm derepression phenotype.RNA Biol. 2021 Jan;18(1):79-92. doi: 10.1080/15476286.2020.1795408. Epub 2020 Aug 30. RNA Biol. 2021. PMID: 32862759 Free PMC article.
-
Metabolic Remodeling during Biofilm Development of Bacillus subtilis.mBio. 2019 May 21;10(3):e00623-19. doi: 10.1128/mBio.00623-19. mBio. 2019. PMID: 31113899 Free PMC article.
References
-
- Hecker M, Volker U. General stress response of Bacillus subtilis and other bacteria. Advances in Microbial Physiology. 2001;44:35–91. - PubMed
-
- Pane-Farre J, Lewis RJ, Stulke J. The RsbRST stress module in bacteria: a signalling system that may interact with different output modules. Journal of Molecular Microbiology and Biotechnology. 2005;9(2):65–76. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

