Normal human gingival epithelial cells sense C. parapsilosis by toll-like receptors and module its pathogenesis through antimicrobial peptides and proinflammatory cytokines
- PMID: 20454633
- PMCID: PMC2862961
- DOI: 10.1155/2010/940383
Normal human gingival epithelial cells sense C. parapsilosis by toll-like receptors and module its pathogenesis through antimicrobial peptides and proinflammatory cytokines
Abstract
This study was designed to investigate the interaction between C. parapsilosis and human epithelial cells using monolayer cultures and an engineered human oral mucosa (EHOM). C. parapsilosis was able to adhere to gingival epithelial cells and to adopt the hyphal form in the presence of serum. Interestingly, when cultured onto the engineered human oral mucosa (EHOM), C. parapsilosis formed small biofilm and invaded the connective tissue. Following contact with C. parapsilosis, normal human gingival epithelial cells expressed high levels of Toll-like receptors (TLR)-2, -4, and -6, but not TLR-9 mRNA. The upregulation of TLRs was paralleled by an increase of IL-1beta, TNFalpha, and IFNgamma mRNA expression, suggesting the involvement of these cytokines in the defense against infection with C. parapsilosis. The active role of epithelial cells in the innate immunity against C. parapsilosis infection was enhanced by their capacity to express high levels of human beta-defensin-1, -2, and -3. The upregulation of proinflammatory cytokines and antimicrobial peptide expression may explain the growth inhibition of C. parapsilosis by the gingival epithelial cells. Overall results provide additional evidence of the involvement of epithelial cells in the innate immunity against C. parapsilosis infections.
Figures

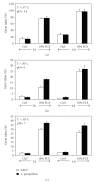


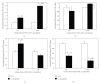



References
-
- Lupetti A, Danesi R, van’t Wout JW, van Dissel JT, Senesi S, Nibbering PH. Antimicrobial peptides: therapeutic potential for the treatment of candida infections. Expert Opinion on Investigational Drugs. 2002;11(2):309–318. - PubMed
-
- Sobel JD. The emergence of non-albicans Candida species as causes of invasive candidiasis and candidemia. Current Infectious Disease Reports. 2006;8(6):427–433. - PubMed
-
- Weems JJ., Jr. Candida parapsilosis: epidemiology, pathogenicity, clinical manifestations, and antimicrobial susceptibility. Clinical Infectious Diseases. 1992;14(3):756–766. - PubMed
-
- Fell JW, Meyer SA. Systematics of yeast species in the Candida parapsilosis group. Mycopathologia et Mycologia Applicata. 1967;32(3):177–193. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

