Agonist anti-GITR monoclonal antibody induces melanoma tumor immunity in mice by altering regulatory T cell stability and intra-tumor accumulation
- PMID: 20454651
- PMCID: PMC2862699
- DOI: 10.1371/journal.pone.0010436
Agonist anti-GITR monoclonal antibody induces melanoma tumor immunity in mice by altering regulatory T cell stability and intra-tumor accumulation
Abstract
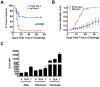
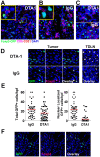
In vivo GITR ligation has previously been shown to augment T-cell-mediated anti-tumor immunity, yet the underlying mechanisms of this activity, particularly its in vivo effects on CD4+ foxp3+ regulatory T cells (Tregs), have not been fully elucidated. In order to translate this immunotherapeutic approach to the clinic it is important gain better understanding of its mechanism(s) of action. Utilizing the agonist anti-GITR monoclonal antibody DTA-1, we found that in vivo GITR ligation modulates regulatory T cells (Tregs) directly during induction of melanoma tumor immunity. As a monotherapy, DTA-1 induced regression of small established B16 melanoma tumors. Although DTA-1 did not alter systemic Treg frequencies nor abrogate the intrinsic suppressive activity of Tregs within the tumor-draining lymph node, intra-tumor Treg accumulation was significantly impaired. This resulted in a greater Teff:Treg ratio and enhanced tumor-specific CD8+ T-cell activity. The decreased intra-tumor Treg accumulation was due both to impaired infiltration, coupled with DTA-1-induced loss of foxp3 expression in intra-tumor Tregs. Histological analysis of B16 tumors grown in Foxp3-GFP mice showed that the majority of GFP+ cells had lost Foxp3 expression. These "unstable" Tregs were absent in IgG-treated tumors and in DTA-1 treated TDLN, demonstrating a tumor-specific effect. Impairment of Treg infiltration was lost if Tregs were GITR(-/-), and the protective effects of DTA-1 were reduced in reconstituted RAG1(-/-) mice if either the Treg or Teff subset were GITR-negative and absent if both were negative. Our results demonstrate that DTA-1 modulates both Teffs and Tregs during effective tumor treatment. The data suggest that DTA-1 prevents intra-tumor Treg accumulation by altering their stability, and as a result of the loss of foxp3 expression, may modify their intra-tumor suppressive capacity. These findings provide further support for the continued development of agonist anti-GITR mAbs as an immunotherapeutic strategy for cancer.
Conflict of interest statement
Figures


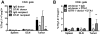


References
-
- Nocentini G, Riccardi C. GITR: a multifaceted regulator of immunity belonging to the tumor necrosis factor receptor superfamily. Eur J Immunol. 2005;35:1016–1022. - PubMed
-
- Kohm AP, Williams JS, Miller SD. Cutting edge: ligation of the glucocorticoid-induced TNF receptor enhances autoreactive CD4+ T cell activation and experimental autoimmune encephalomyelitis. J Immunol. 2004;172:4686–4690. - PubMed
-
- Kanamaru F, Youngnak P, Hashiguchi M, Nishioka T, Takahashi T, et al. Costimulation via glucocorticoid-induced TNF receptor in both conventional and CD25+ regulatory CD4+ T cells. J Immunol. 2004;172:7306–7314. - PubMed
-
- Ronchetti S, Zollo O, Bruscoli S, Agostini M, Bianchini R, et al. GITR, a member of the TNF receptor superfamily, is costimulatory to mouse T lymphocyte subpopulations. Eur J Immunol. 2004;34:613–622. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R25 CA020449/CA/NCI NIH HHS/United States
- K12 CA120121/CA/NCI NIH HHS/United States
- K08CA127143-01/CA/NCI NIH HHS/United States
- K08CA10260/CA/NCI NIH HHS/United States
- P01 CA059350/CA/NCI NIH HHS/United States
- CA56821/CA/NCI NIH HHS/United States
- T32 CA09149-30/CA/NCI NIH HHS/United States
- CA33049/CA/NCI NIH HHS/United States
- K08 CA127143/CA/NCI NIH HHS/United States
- P01 CA047179/CA/NCI NIH HHS/United States
- T32 CA009149/CA/NCI NIH HHS/United States
- P01 CA033049/CA/NCI NIH HHS/United States
- K12 CA120121-01/CA/NCI NIH HHS/United States
- CA59350/CA/NCI NIH HHS/United States
- CA47179/CA/NCI NIH HHS/United States
- R01 CA056821/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

