Resolution and polarization distribution in cryogenic DNP/MAS experiments
- PMID: 20454732
- PMCID: PMC4085575
- DOI: 10.1039/c003763j
Resolution and polarization distribution in cryogenic DNP/MAS experiments
Abstract
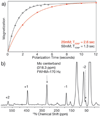
This contribution addresses four potential misconceptions associated with high-resolution dynamic nuclear polarization/magic angle spinning (DNP/MAS) experiments. First, spectral resolution is not generally compromised at the cryogenic temperatures at which DNP experiments are performed. As we demonstrate at a modest field of 9 T (380 MHz (1)H), 1 ppm linewidths are observed in DNP/MAS spectra of a membrane protein in its native lipid bilayer, and <0.4 ppm linewidths are reported in a crystalline peptide at 85 K. Second, we address the concerns about paramagnetic broadening in DNP/MAS spectra of proteins by demonstrating that the exogenous radical polarizing agents utilized for DNP are distributed in the sample in such a manner as to avoid paramagnetic broadening and thus maintain full spectral resolution. Third, the enhanced polarization is not localized around the polarizing agent, but rather is effectively and uniformly dispersed throughout the sample, even in the case of membrane proteins. Fourth, the distribution of polarization from the electron spins mediated via spin diffusion between (1)H-(1)H strongly dipolar coupled spins is so rapid that shorter magnetization recovery periods between signal averaging transients can be utilized in DNP/MAS experiments than in typical experiments performed at ambient temperature.
Figures




References
-
- Carver TR, Slichter CP. Phys. Rev. 1956;102:975–980.
-
- Rosay M, Lansing JC, Haddad KC, Bachovchin WW, Herzfeld J, Temkin RJ, Griffin RG. J. Am. Chem. Soc. 2003;125:13626–13627. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

