Evaluation of enhanced condensational growth (ECG) for controlled respiratory drug delivery in a mouth-throat and upper tracheobronchial model
- PMID: 20454837
- PMCID: PMC2916966
- DOI: 10.1007/s11095-010-0165-z
Evaluation of enhanced condensational growth (ECG) for controlled respiratory drug delivery in a mouth-throat and upper tracheobronchial model
Abstract
Purpose: The objective of this study is to evaluate the effects of enhanced condensational growth (ECG), as a novel inhalation drug delivery method, on nano-aerosol deposition in a mouth-throat (MT) and upper tracheobronchial (TB) model using in vitro experiments and computational fluid dynamics (CFD) simulations.
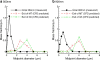
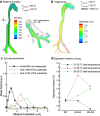
Methods: Separate streams of nebulized nano-aerosols and saturated humidified air (39 degrees C-ECG; 25 degrees C-control) were combined as they were introduced into a realistic MT-TB geometry. Aerosol deposition was determined in the MT, generations G0-G2 (trachea-lobar bronchi) and G3-G5 and compared to CFD simulations.
Results: Using ECG conditions, deposition of 560 and 900 nm aerosols was low in the MT region of the MT-TB model. Aerosol drug deposition in the G0-G2 and G3-G5 regions increased due to enhanced condensational growth compared to control. CFD-predicted depositions were generally in good agreement with the experimental values.
Conclusions: The ECG platform appears to offer an effective method of delivering nano-aerosols through the extrathoracic region, with minimal deposition, to the tracheobronchial airways and beyond. Aerosol deposition is then facilitated as enhanced condensational growth increases particle size. Future studies will investigate the effects of physio-chemical drug properties and realistic inhalation profiles on ECG growth characteristics.
Figures


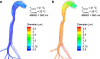


Similar articles
-
Use of computational fluid dynamics deposition modeling in respiratory drug delivery.Expert Opin Drug Deliv. 2019 Jan;16(1):7-26. doi: 10.1080/17425247.2019.1551875. Epub 2018 Dec 10. Expert Opin Drug Deliv. 2019. PMID: 30463458 Free PMC article. Review.
-
Characterization of respiratory drug delivery with enhanced condensational growth using an individual path model of the entire tracheobronchial airways.Ann Biomed Eng. 2011 Mar;39(3):1136-53. doi: 10.1007/s10439-010-0223-z. Epub 2010 Dec 9. Ann Biomed Eng. 2011. PMID: 21152983 Free PMC article.
-
Improving the lung delivery of nasally administered aerosols during noninvasive ventilation-an application of enhanced condensational growth (ECG).J Aerosol Med Pulm Drug Deliv. 2011 Apr;24(2):103-18. doi: 10.1089/jamp.2010.0849. Epub 2011 Mar 16. J Aerosol Med Pulm Drug Deliv. 2011. PMID: 21410327 Free PMC article.
-
Effects of the laryngeal jet on nano- and microparticle transport and deposition in an approximate model of the upper tracheobronchial airways.J Appl Physiol (1985). 2008 Jun;104(6):1761-77. doi: 10.1152/japplphysiol.01233.2007. Epub 2008 Apr 3. J Appl Physiol (1985). 2008. PMID: 18388247
-
Pediatric in vitro and in silico models of deposition via oral and nasal inhalation.J Aerosol Med Pulm Drug Deliv. 2014 Jun;27(3):149-69. doi: 10.1089/jamp.2013.1075. J Aerosol Med Pulm Drug Deliv. 2014. PMID: 24870701 Review.
Cited by
-
Use of computational fluid dynamics deposition modeling in respiratory drug delivery.Expert Opin Drug Deliv. 2019 Jan;16(1):7-26. doi: 10.1080/17425247.2019.1551875. Epub 2018 Dec 10. Expert Opin Drug Deliv. 2019. PMID: 30463458 Free PMC article. Review.
-
A Review of Respiratory Anatomical Development, Air Flow Characterization and Particle Deposition.Int J Environ Res Public Health. 2020 Jan 7;17(2):380. doi: 10.3390/ijerph17020380. Int J Environ Res Public Health. 2020. PMID: 31935991 Free PMC article. Review.
-
Drug-targeting methodologies with applications: A review.World J Clin Cases. 2014 Dec 16;2(12):742-56. doi: 10.12998/wjcc.v2.i12.742. World J Clin Cases. 2014. PMID: 25516850 Free PMC article. Review.
-
Asymmetric lung increases particle filtration by deposition.Sci Rep. 2023 Jun 3;13(1):9040. doi: 10.1038/s41598-023-36176-3. Sci Rep. 2023. PMID: 37270569 Free PMC article.
-
Devices for Improved Delivery of Nebulized Pharmaceutical Aerosols to the Lungs.J Aerosol Med Pulm Drug Deliv. 2019 Oct;32(5):317-339. doi: 10.1089/jamp.2018.1508. Epub 2019 Jul 9. J Aerosol Med Pulm Drug Deliv. 2019. PMID: 31287369 Free PMC article. Review.
References
-
- Borgstrom L, Olsson B, Thorsson L. Degree of throat deposition can explain the variability in lung deposition of inhaled drugs. J Aerosol Med. 2006;19:473–83. - PubMed
-
- Newman SP. A comparison of lung deposition patterns between different asthma inhalers. J Aerosol Med. 1995;8(Suppl 3):S21–26. discussion S27. - PubMed
-
- Leach CL, Davidson PJ, Bouhuys A. Improved airway targeting with the CFC-free HFA-beclomethasone metered-dose inhaler compared with CFC-beclomethasone. Eur Respir J. 1998;12:1346–53. - PubMed
-
- Smaldone GC. Advances in aerosols: adult respiratory disease. J Aerosol Med. 2006;19:36–46. - PubMed
-
- Byron PR. Drug delivery devices: issues in drug development. Proc Am Thorac Soc. 2004;1:321–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

