Efficacy and safety of a new 20% immunoglobulin preparation for subcutaneous administration, IgPro20, in patients with primary immunodeficiency
- PMID: 20454851
- PMCID: PMC2935975
- DOI: 10.1007/s10875-010-9423-4
Efficacy and safety of a new 20% immunoglobulin preparation for subcutaneous administration, IgPro20, in patients with primary immunodeficiency
Abstract
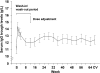
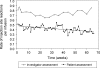
Subcutaneous human IgG (SCIG) therapy in primary immunodeficiency (PID) offers sustained IgG levels throughout the dosing cycle and fewer adverse events (AEs) compared to intravenous immunoglobulin (IVIG). A phase I study showed good local tolerability of IgPro20, a new 20% liquid SCIG stabilized with L-proline. A prospective, open-label, multicenter, single-arm, phase III study evaluated the efficacy and safety of IgPro20 in patients with PID over 15 months. Forty-nine patients (5-72 years) previously treated with IVIG received weekly subcutaneous infusions of IgPro20. The mean serum IgG level was 12.5 g/L. No serious bacterial infections were reported. There were 96 nonserious infections (rate 2.76/patient per year). The rate of days missed from work/school was 2.06/patient per year, and the rate of hospitalization was 0.2/patient per year. Ninety-nine percent of AEs were mild or moderate. No serious, IgPro20-related AEs were reported. IgPro20 effectively protected patients with PID against infections and maintained serum IgG levels without causing unexpected AEs.
Figures
Comment in
-
Incidence of infection is inversely related to steady-state (trough) serum IgG level in studies of subcutaneous IgG in PIDD.J Clin Immunol. 2011 Oct;31(5):924-6. doi: 10.1007/s10875-011-9546-2. Epub 2011 Jun 4. J Clin Immunol. 2011. PMID: 21643892
References
-
- Ochs HD, Smith CIE, Puck JM. Primary immunodeficiency diseases: a molecular and genetic approach. Second. New York: Oxford University Press Inc, USA; 2007.
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

