Immunological origin and functional properties of catalytic autoantibodies to amyloid beta peptide
- PMID: 20454852
- PMCID: PMC3147076
- DOI: 10.1007/s10875-010-9414-5
Immunological origin and functional properties of catalytic autoantibodies to amyloid beta peptide
Abstract
Objectives: Objectives The objectives of this study are to (1) evaluate the ability of the immune system to synthesize specific antibodies that catalyze the degradation of amyloid beta peptide (Abeta) and to (2) evaluate the prospect of developing a catalytic IVIG (CIVIG) formulation for therapy of Alzheimer's disease (AD).
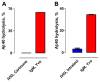
Conclusions: Polyclonal autoantibodies from humans without dementia hydrolyzed Abeta specifically. The catalytic activity improved as a function of age. Patients with AD produced catalytic antibodies at increased levels. IgM-class antibodies expressed the activity at levels superior to IgGs. Production of catalytic autoantibodies appears to be an innate immunity function with adaptive improvements occurring upon Abeta overexpression, which suggests a beneficial function of the catalytic activity. The catalytic autoantibodies impeded Abeta aggregation, dissolved preformed Abeta aggregates, and inhibited Abeta cytotoxicity in tissue culture. Recombinant catalytic antibodies from a human library have been identified, validating the phenomenon of antibody-catalyzed Abeta cleavage. As a single catalyst molecule inactivates multiple Abeta molecules, catalytic antibodies may clear Abeta efficiently. IVIG did not cleave Abeta, indicating the importance of purification procedures that maintain catalytic site integrity. Traditional Abeta-binding antibodies form immune complexes that can induce inflammatory reaction and vascular dysfunction. Catalysts do not form stable immune complexes, minimizing these risks. Criteria appropriate for developing a CIVIG formulation with potential therapeutic utility are discussed, including isolation of the Abeta-specific catalytic subsets present in IgM and IgG from human blood.
Figures


References
-
- Vani J, Elluru S, Negi VS, Lacroix-Desmazes S, Kazatchkine MD, Bayary J, et al. Role of natural antibodies in immune homeostasis: IVIg perspective. Autoimmun Rev. 2008;7:440–4. - PubMed
-
- Paul S, Volle DJ, Beach CM, Johnson DR, Powell MJ, Massey RJ. Catalytic hydrolysis of vasoactive intestinal peptide by human autoantibody. Science. 1989;244:1158–62. - PubMed
-
- Paul S, Nishiyama Y, Planque S, Taguchi H. Theory of proteolytic antibody occurrence. Immunol Lett. 2006;103:8–16. - PubMed
-
- Paul S, Nishiyama Y, Planque S, Karle S, Taguchi H, Hanson C, et al. Antibodies as defensive enzymes. Springer Semin Immunopathol. 2005;26:485–503. - PubMed
-
- Planque S, Mitsuda Y, Taguchi H, Salas M, Morris MK, Nishiyama Y, et al. Characterization of gp120 hydrolysis by IgA antibodies from humans without HIV infection. AIDS Res Hum Retroviruses. 2007;23:1541–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

