Glial versus melanocyte cell fate choice: Schwann cell precursors as a cellular origin of melanocytes
- PMID: 20454996
- PMCID: PMC11115498
- DOI: 10.1007/s00018-010-0390-y
Glial versus melanocyte cell fate choice: Schwann cell precursors as a cellular origin of melanocytes
Abstract
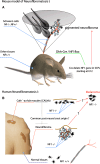
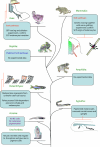
Melanocytes and Schwann cells are derived from the multipotent population of neural crest cells. Although both cell types were thought to be generated through completely distinct pathways and molecular processes, a recent study has revealed that these different cell types are intimately interconnected far beyond previously postulated limits in that they share a common post-neural crest progenitor, i.e. the Schwann cell precursor. This finding raises interesting questions about the lineage relationships of hitherto unrelated cell types such as melanocytes and Schwann cells, and may provide clinical insights into mechanisms of pigmentation disorders and for cancer involving Schwann cells and melanocytes.
Figures


References
-
- Adameyko I, Lallemend F, Aquino JB, Pereira JA, Topilko P, Muller T, Fritz N, Beljajeva A, Mochii M, Liste I, Usoskin D, Suter U, Birchmeier C, Ernfors P. Schwann cell precursors from nerve innervation are a cellular origin of melanocytes in skin. Cell. 2009;139:366–379. doi: 10.1016/j.cell.2009.07.049. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

