Knockdown of CypA inhibits interleukin-8 (IL-8) and IL-8-mediated proliferation and tumor growth of glioblastoma cells through down-regulated NF-κB
- PMID: 20454998
- PMCID: PMC2995866
- DOI: 10.1007/s11060-010-0220-y
Knockdown of CypA inhibits interleukin-8 (IL-8) and IL-8-mediated proliferation and tumor growth of glioblastoma cells through down-regulated NF-κB
Abstract
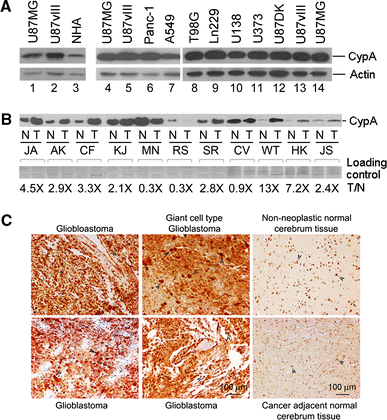
Although cyclophilin A (CypA) has been reported to be over-expressed in cancer cells and solid tumors, its expression and role in glioblastomas have not been studied. Herein, we show that expression of CypA in human glioblastoma cell lines and tissues is significantly higher than in normal human astrocytes and normal counterparts of brain tissue. To determine the role of over-expressed CypA in glioblastoma, stable RNA interference (RNAi)-mediated knockdown of CypA (CypA KD) was performed in gliobastoma cell line U87vIII (U87MG · ΔEGFR). CypA KD stable single clones decrease proliferation, infiltration, migration, and anchorage-independent growth in vitro and with slower growth in vivo as xenografts in immunodeficient nude mice. We have also observed that knockdown of CypA inhibits expression of interleukin-8 (IL-8), a tumorigenic and proangiogenic cytokine. Conversely, enforced expression of CypA in the CypA KD cell line, Ud-12, markedly enhanced IL-8 transcripts and restored Ud-12 proliferation, suggesting that CypA-mediated IL-8 production provides a growth advantage to glioblastoma cells. CypA knockdown-mediated inhibition of IL-8 is due to reduced activity of NF-κB, which is one of the major transcription factors regulating IL-8 expression. These results not only establish the relevance of CypA to glioblastoma growth in vitro and in vivo, but also suggest that small interfering RNA-based CypA knockdown could be an effective therapeutic approach against glioblastomas.
Figures






Similar articles
-
Downregulation of cyclophilin A by siRNA diminishes non-small cell lung cancer cell growth and metastasis via the regulation of matrix metallopeptidase 9.BMC Cancer. 2012 Oct 2;12:442. doi: 10.1186/1471-2407-12-442. BMC Cancer. 2012. PMID: 23031673 Free PMC article.
-
Stable RNA interference-mediated suppression of cyclophilin A diminishes non-small-cell lung tumor growth in vivo.Cancer Res. 2005 Oct 1;65(19):8853-60. doi: 10.1158/0008-5472.CAN-05-1219. Cancer Res. 2005. PMID: 16204056
-
Analysis of interleukin-6 gene expression in primary human gliomas, glioblastoma xenografts, and glioblastoma cell lines.Brain Tumor Pathol. 2001;18(1):13-21. doi: 10.1007/BF02478920. Brain Tumor Pathol. 2001. PMID: 11517969
-
Cyclophilin A Enhances Cell Proliferation and Xenografted Tumor Growth of Early Gastric Cancer.Dig Dis Sci. 2015 Sep;60(9):2700-11. doi: 10.1007/s10620-015-3694-9. Epub 2015 May 26. Dig Dis Sci. 2015. PMID: 26008617
-
[Progress of CypA and lung cancer-related research].Zhongguo Fei Ai Za Zhi. 2010 Aug;13(8):827-31. doi: 10.3779/j.issn.1009-3419.2010.08.15. Zhongguo Fei Ai Za Zhi. 2010. PMID: 20704827 Free PMC article. Review. Chinese.
Cited by
-
Targeting oncomiRNAs and mimicking tumor suppressor miRNAs: Νew trends in the development of miRNA therapeutic strategies in oncology (Review).Int J Oncol. 2016 Jul;49(1):5-32. doi: 10.3892/ijo.2016.3503. Epub 2016 May 4. Int J Oncol. 2016. PMID: 27175518 Free PMC article. Review.
-
Elevated plasma 20S proteasome chymotrypsin-like activity is correlated with IL-8 levels and associated with an increased risk of death in glial brain tumor patients.PLoS One. 2020 Sep 4;15(9):e0238406. doi: 10.1371/journal.pone.0238406. eCollection 2020. PLoS One. 2020. PMID: 32886667 Free PMC article.
-
Cyclophilin A enhances cell proliferation and tumor growth of liver fluke-associated cholangiocarcinoma.Mol Cancer. 2011 Aug 26;10:102. doi: 10.1186/1476-4598-10-102. Mol Cancer. 2011. PMID: 21871105 Free PMC article.
-
Ubiquitin-specific protease 4 promotes hepatocellular carcinoma progression via cyclophilin A stabilization and deubiquitination.Cell Death Dis. 2018 Feb 2;9(2):148. doi: 10.1038/s41419-017-0182-5. Cell Death Dis. 2018. PMID: 29396555 Free PMC article.
-
Novel role for cyclophilin A in regulation of chondrogenic commitment and endochondral ossification.Mol Cell Biol. 2015 Jun;35(12):2119-30. doi: 10.1128/MCB.01414-14. Epub 2015 Apr 13. Mol Cell Biol. 2015. PMID: 25870110 Free PMC article.

