The use of small molecule high-throughput screening to identify inhibitors of the proteinase 3-NB1 interaction
- PMID: 20456416
- PMCID: PMC2909422
- DOI: 10.1111/j.1365-2249.2010.04174.x
The use of small molecule high-throughput screening to identify inhibitors of the proteinase 3-NB1 interaction
Abstract
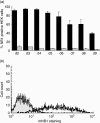
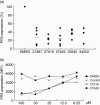
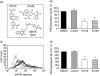
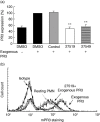
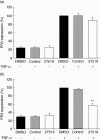
Anti-neutrophil cytoplasmic antibodies (ANCA) to proteinase 3 (PR3) are found in patients with small-vessel vasculitis. PR3-ANCA bind strongly to membrane PR3 (mPR3) that is presented by the NB1 receptor. We performed high-throughput screening using a small molecule library to identify compounds that inhibit PR3-NB1 binding. We established a human embryonic kidney (HEK293) cell-based system, where approximately 95 +/- 2% of the NB1-transfected cells expressed the NB1 receptor on the cell surface. Addition of 0.1 microg/ml human PR3 to 10(4) NB1-expressing HEK293 cells resulted in PR3 binding that was detected by immunofluorescence using a fluorescence plate reader assay. We identified 13 of 20 000 molecules that inhibited PR3 binding by >70%. Seven of 13 substances showed reproducible inhibition in four additional validation experiments. Two selected compounds (27519 and 27549) demonstrated a dose-dependent inhibition over a range from 6.25 to 100 microM as measured by the plate reader assay. We used flow cytometry as a second assay, and found that both compounds reproducibly inhibited PR3 binding to NB1-transfected HEK293 cells at 50 microM (inhibition to 42 +/- 4% with compound 27519 and to 47 +/- 6% with compound 27549 compared to the dimethylsulphoxide control). Furthermore, compounds 27519 and 27549 also inhibited binding of exogenous PR3 to human neutrophils. In contrast, the compounds did not decrease mPR3 expression on resting neutrophils, but reduced the tumour necrosis factor-alpha-mediated mPR3 increase on NB1(pos) neutrophils when present continuously during the assay. The findings suggest that small inhibitory compounds provide a potential therapeutic tool to reduce mPR3 by preventing its binding to NB1.
Figures
Similar articles
-
Complement receptor Mac-1 is an adaptor for NB1 (CD177)-mediated PR3-ANCA neutrophil activation.J Biol Chem. 2011 Mar 4;286(9):7070-81. doi: 10.1074/jbc.M110.171256. Epub 2010 Dec 30. J Biol Chem. 2011. PMID: 21193407 Free PMC article.
-
NB1 mediates surface expression of the ANCA antigen proteinase 3 on human neutrophils.Blood. 2007 May 15;109(10):4487-93. doi: 10.1182/blood-2006-10-055327. Epub 2007 Jan 23. Blood. 2007. PMID: 17244676
-
A hydrophobic patch on proteinase 3, the target of autoantibodies in Wegener granulomatosis, mediates membrane binding via NB1 receptors.J Biol Chem. 2008 Dec 19;283(51):35976-82. doi: 10.1074/jbc.M806754200. Epub 2008 Oct 14. J Biol Chem. 2008. PMID: 18854317
-
Membrane-bound proteinase 3 and its receptors: relevance for the pathogenesis of Wegener's Granulomatosis.Autoimmun Rev. 2009 May;8(6):510-4. doi: 10.1016/j.autrev.2008.01.003. Epub 2009 Feb 4. Autoimmun Rev. 2009. PMID: 19185066 Review.
-
Membrane proteinase 3 expression on resting neutrophils as a pathogenic factor in PR3-ANCA-associated vasculitis.Clin Exp Rheumatol. 2003 Nov-Dec;21(6 Suppl 32):S64-8. Clin Exp Rheumatol. 2003. PMID: 14740429 Review.
Cited by
-
Development of a Novel Backbone Cyclic Peptide Inhibitor of the Innate Immune TLR/IL1R Signaling Protein MyD88.Sci Rep. 2018 Jun 21;8(1):9476. doi: 10.1038/s41598-018-27773-8. Sci Rep. 2018. PMID: 29930295 Free PMC article.
-
Interaction of serine proteases from polymorphonuclear leucocytes with the cell surface and heparin.Inflammation. 2012 Feb;35(1):81-8. doi: 10.1007/s10753-011-9292-x. Inflammation. 2012. PMID: 21246269
-
Characterization of the CD177 interaction with the ANCA antigen proteinase 3.Sci Rep. 2017 Feb 27;7:43328. doi: 10.1038/srep43328. Sci Rep. 2017. PMID: 28240246 Free PMC article.
-
How anti-neutrophil cytoplasmic autoantibodies activate neutrophils.Clin Exp Immunol. 2012 Sep;169(3):220-8. doi: 10.1111/j.1365-2249.2012.04615.x. Clin Exp Immunol. 2012. PMID: 22861361 Free PMC article. Review.
-
Small-molecule screening identifies modulators of aquaporin-2 trafficking.J Am Soc Nephrol. 2013 Apr;24(5):744-58. doi: 10.1681/ASN.2012030295. Epub 2013 Apr 4. J Am Soc Nephrol. 2013. PMID: 23559583 Free PMC article.
References
-
- Niles JL, McCluskey RT, Ahmad MF, Arnaout MA. Wegener's granulomatosis autoantigen is a novel neutrophil serine proteinase. Blood. 1989;74:1888–93. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

